Blood Glucose Sensor
Description:
The Extracorporeal Blood Glucose sensor will utilize a glucose – glucose oxidase reaction to generate a measurable current on a circuit system. This allows us to know if an officer is bleeding in the region surrounding the sensor. Updates will become available as we begin testing.
Updates:
April 18
Continuing testing of different concentrations of glucose, we wanted to ensure that our extracorporeal blood sensor would not send false-positives to dispatch by interacting with sweat (glucose is present in sweat at roughly 6 mg/dL). Therefore, we tested this concentration of glucose on our electrode setup, and saw insignificant changes in voltage output (see graph).
We also have shown stability of the enzyme on our electrode system for two months without substantial degradation (see graph).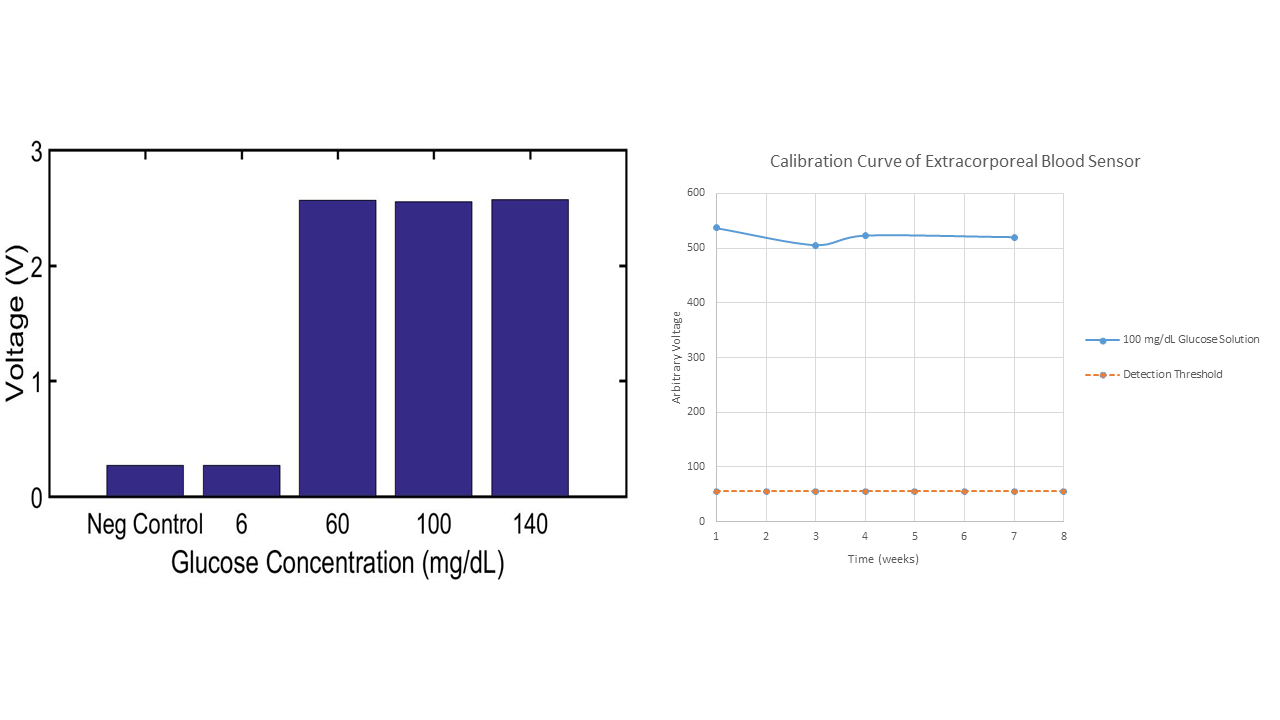 March 26
March 26
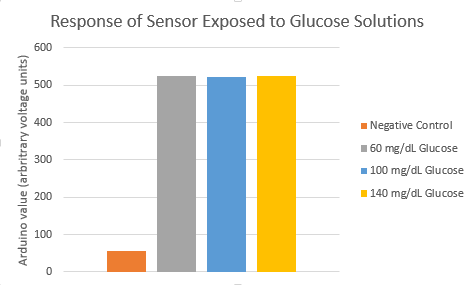
We conducted experiments with differing concentrations of glucose in solution. This was done to validate our device sensitivity over a wide range of possible blood glucose values, as most officers will not have the average glucose concentration when in threatening situations. The graph below shows the results of three concentrations of glucose (low, average, high), as well as a negative control, for the sensor. One can see that there is no substantial difference in signal collected from different concentrations of solution. Moreover, all of these are substantially higher than the value of the negative control, allowing for a large thresholding window. The only noticeable differences were a slightly longer time (2-3 minutes) for signal detection in the 60 mg/dL glucose solution.
March 21
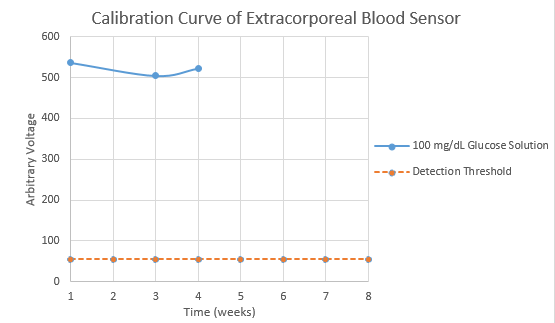
We have shown efficacy of the extracorporeal glucose sensor over a period of a month. Considering the relative simplicity and the low cost of the module, this is a very encouraging result. We will continue to test the longevity of the enzyme.
March 14 (and onward)
We have begun a calibration curve for testing the electric output of the reaction as a function of time. This will allow us to set alerting thresholds in our algorithm, as well as show us how long the sensors can last before needing to be replaced (as the enzyme will degrade over time). The graph below will be updated as we continue to test longer time periods. We also plan to vary the concentration of glucose in future tests to know the range in which we can detect its presence.
February 21
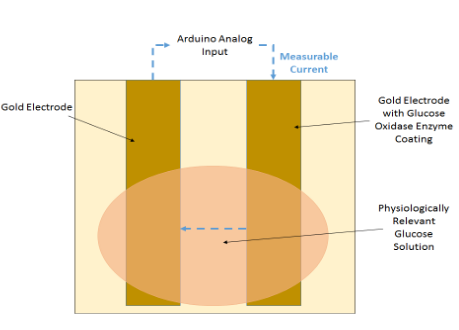
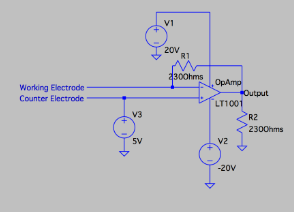
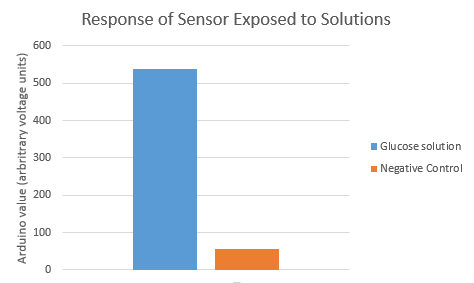
We demonstrated a proof-of-concept for the blood glucose sensor. We first set up a two-electrode system, where both electrodes are gold and one electrode is coated in a glucose oxidase enzyme. We then exposed both electrodes to a 100 mg/dL glucose solution (which mimics the average in vivo concentration of glucose in blood). The addition of glucose allows an electrochemically-active reaction to occur on the electrodes. For validity purposes, we also exposed the electrodes to a glucose-negative solution, to ensure that the reaction itself is changing the sensor readings and not the electrodes being touched by a liquid. Using Arduino, we measured the electric signal generated as a result of this reaction.
The electrode setup and circuit diagram can be seen below.
We received very encouraging data from this setup. The results are shown in the graph below. This shows that we can harness the electrochemical potential of this reaction to efficiently tell whether or not an officer is bleeding near the sensor.
©2026 Vanderbilt University ·
Site Development: University Web Communications