Results
Proof of Concept
UV-Vis-NIR Spectrophotometry Trials
To calibrate our device, we needed to show lactic acid detection by absorbance using an IR spectrophotometer. Attempts to detect varied concentrations in water and research-grade acetone are seen in figures 1.
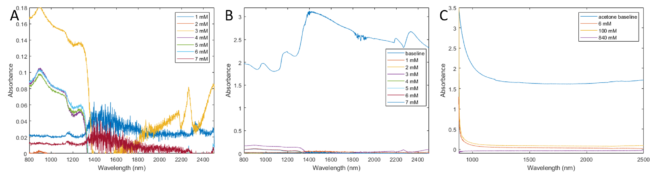
A) Lactate in water, baseline-adjusted
B) Lactate in water, baseline-adjusted, with water absorbance overlayed
C) Lactate in acetone, baseline-adjusted, with acetone absorbance overlayed
Device Verification
To prove that the LED-photoconductor circuit worked as designed, the two circuits were put close together (<1cm) and the photoconductor output was recorded. A Fourier transform of the output showed strong signal at the LED pulse frequency as seen in figure 2.
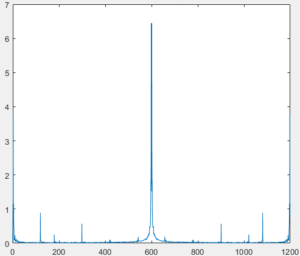
Conclusion
In the given time frame, a construct of a novel circuit for detection of lactate was developed. We were able to come to the following conclusions:
- Our device can detect changes in absorbance, as seen in figure 4.
- Further optimization needs to be done to finetune the spectroscopy for various concentrations of lactate.
- The lactate signal was virtually undetectable relative to water and other hydrogenated solvents (i.e., low signal-to-noise ratio [SNR]).
- Use a deuterated solvent to acquire a better signal and improve SNR.