Definition/Background
Gene therapy is an experimental treatment that targets and fixes abnormalities in a patient’s genetic sequence to eradicate disease (National Institutes of Health 2017). This interaction with the human genome can take a number of different forms; researchers are currently developing techniques that would allow them to replace mutated genes with a healthy copy, inactivate mutated genes, and/or introduce new genes into the body that could help fight disease (National Institutes of Health 2017). However, gene therapy is relatively new, and researchers have only created successful treatment approaches for a few single-gene disorders, including Gaucher’s disease and phenylketonuria (Hunt 2008). Nonetheless, these monogenetic diseases rank second compared to cancer as the most common disease treated with gene therapy (Wirth 2008). Gene therapy is still considered high-risk and is being studied around the world to ensure it is an effective and safe treatment.
Since it is so new to health care providers, federal agencies of different nations have defined the technical measures of gene therapy differently. For example, the European Medicines Agency (EMA) defines gene therapy as:
a biological medicinal product which fulfils the following two characteristics: (a) it contains an active substance which contains or consists of a recombinant nucleic acid used in or administered to human beings with a view to regulating, repairing, replacing, adding or deleting a genetic sequence; (b) its therapeutic, prophylactic or diagnostic effect relates directly to the recombinant nucleic acid sequence it contains, or to the product of genetic expression of this sequence (Wirth 2013)
Whereas, the U.S. Food and Drug Administration (FDA) defines gene therapy as:
a product that mediate their effects by transcription and/or translation of transferred genetic material and/or by integrating into the host genome and that are administered as nucleic acids, viruses, or genetically engineered microorganisms. The products may be used to modify cells in vivo or transferred to cells ex vivo prior to administration to the recipient (Wirth 2013)
While the definitions seem similar on the surface, a few words and phrases differentiate how Europe and the U.S. view gene therapy. For instance, the EMA refers to the prophylactic nature of the treatment; however, the FDA definition does not mention these same benefits. The FDA definition seems to focus more on how gene therapy can be studied further (i.e. through cells in vivo or ex vivo). This analysis seems to match the implementation of gene therapy in each of these nations as well. The U.S. has yet to approve gene therapy as a valid treatment course, hinging on the fact that more research has to be done, while Europe approved Glybera – a gene therapy technique, in November 2012 (DeWeerdt 2014).
As gene therapy continues to develop, there are two distinct types of therapy that have to be considered – somatic and germ-line (Wirth 2013). The difference between the two is simple, but the implications of using one over the other create long-lasting impacts. Somatic gene therapy is when genetic material is inserted into target cells that cannot pass on their genetic material to future generations (Wirth 2013). Whereas, germ-line therapy modifies genes that will be passed on to the next generation (think egg and sperm cells) (Wirth 2013). Current U.S. legislation only allows for somatic therapy because germ-line therapy leads to ethical questions related to eugenics, which will be discussed later on (Wirth 2013).
While somatic and germ-line therapies target different cells types, the mechanism by which the treatment is administered is essentially the same. The overall goal of gene therapy is to replace mutated DNA to improve clinical status of the patient (Patil 2012). To reach this goal, most therapies begin by inserting a normal or modified version of the target genetic sequence into a viral vector (Hunt 2008). Viral vectors are viruses with artificially incorporated DNA (Mestrovic 2015). The vectors can then transfer that genetic material into the nucleus of a target cell, essentially acting as genetic shuttles (Patil 2012). Inserting the new genes will then alter protein or hormone expression in the target cell, which creates the desired therapeutic effect (Patil 2012). This process is illustrated in Figure 1 depicted below.
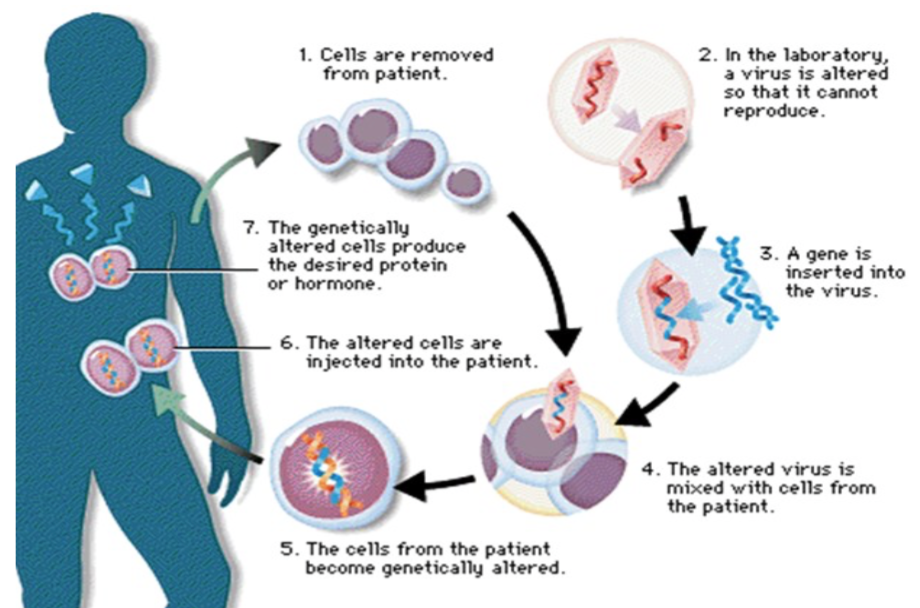
Fig. 1 – Flow chart of gene therapy mechanism (Patil 2012)
There are multiple ways the inserted genetic material can then interact with the DNA of the target cell (Hunt 2008). For example, there is random insertion of a normal gene into the genome, which is most common, replacement of an abnormal gene with a normal one, repair of an abnormal gene, or altering the regulation of a certain genetic sequence (Patil 2008). There are also nonviral methods of gene therapy that do not use a viral vector (Cotrim 2008). While a nonviral approach is typically safer, this method is inefficient at transferring genes and has not been as widely used or studied (Cotrim 2008).
Historical Context
Even though the application of gene therapy is relatively new, the discussion surrounding the technique has been active for over forty years. In 1972, twenty years before the first human gene therapy trial would even begin, Theodore Friedmann hoped to establish criteria that would guide the creation and application of future gene therapy techniques (Wirth 2013). He argued that all further attempts at creating this treatment should be opposed because of inadequate knowledge of gene regulation, rudimentary understating of the connection between diseased state and molecular abnormalities, and lack of information on short- or long-term effects of gene therapy (Friedmann 1972). Of course, this was still in 1972, and American scientists have since made great progress in their knowledge of diseased states as well as basic information regarding genetic regulation, replication, et cetera.
Moving on from Friedmann’s skepticism in the 70s, progress was slow but picked up in the mid-80s when a retrovirus vector system was able to effectively insert foreign DNA into mammalian chromosomes (Cepko et al. 1984). Once this viral method of gene transfer was found, gene therapy took off in the 1990s, and “the hype was astronomical” (Obasogie 2009). In fact, the promise that it could cure some of the most prevalent diseases as well as make billions of dollars in profit lead to several companies investing millions of dollars in technology for the therapy (Obasogie 2009). The first clinical gene therapy trial was conducted by Rosenberg et al. in 1990, which helped researchers better understand the kinds of cancer cells they should be targeting (tumor infiltrating lymphocytes) and establish that the viral vector system created in the 80s could be safely used in humans (Rosenberg et al. 1990).
Since this first clinical trial, there has been much attention and money given to the field by both the private- and public-sector (Cotrim 2008). Additionally, there have been numerous animal-model studies that have proven the efficacy of clinical applications. For example, in 2002, a study showed that sickle-cell disease could be treated in mice through gene therapy (Wilson 2002). At present, the latest research on gene therapy was performed by scientist Tom Whipple in patients with advanced Non-Hodgkin’s lymphoma (Whipple 2017). The clinical trial showed “extraordinary” results where about 80% of 101 patients had their cancer shrink by 50% or more (Whipple 2017). A third of these patients had lymphoma that was not affected by any other form of treatment (Whipple 2017). This kind of promise keeps both scientists and investors interested in the potential of gene therapy.
Controversy/Perspectives
Even though the promise of gene therapy is immense, its development has not been without controversy. The biggest scandal occurred in 1999 when a teenager named Jesse Gelsinger died during a clinical trial at the University of Pennsylvania (Obasogie 2009). Gelsinger suffered from ornithine transcarbamylase deficiency (OTCD), which inhibits the body’s ability to break down ammonia (OTCD 2017) While it can be managed, the most severe cases are often fatal (OTCD 2017). He enrolled in the OTCD clinical trial that tested a new mechanism to deliver healthy OTC genes to patients’ livers (Obasogie 2009). While Gelsinger would not have benefited from the studies himself, he enrolled in the trial as an altruistic act with the hopes that the knowledge gained would benefit those with more severe cases of OTCD (Obasogie 2009). Not long after the doctors administered the replacement genes, Jesse’s ammonia level skyrocketed, leaving him with irreparable brain damage, organ failure, and in a coma (Obasogie 2009). He died a few days later when his parents removed him from life support (Obasogie 2009). This instance created lasting repercussions for the progress of gene therapy research. In this particular case, critics scrutinized the financial interests of the scientists behind the OTCD clinical trials and how it affected the recruitment of subjects and the progress of the study (Wilson 2010). This sparked a legacy of deeper investigation into researchers’ financial interests in their human trials both nationwide and at the University of Pennsylvania (Wilson 2010).
Aside from this discrete incident, there are many theoretical and controversial questions left unanswered about the potential of gene therapy. The most prevalent questions center around germ-line cell therapy. Since germ-line therapy would create genetic changes that are passed on to future generations, there is a fear that errors or potential side effects from gene therapy would be further propagated (Diehl 2017). Scientists like Friedmann, mentioned earlier, would caution researchers to have a better knowledge of the possible consequences before proceeding. In fact, Matthew Porteus from Stanford University theorized four possible ways to minimize risk in performing gene therapy:
1) continued improvements in vector design, 2) buffer the genome from the effect of viral integrations, 3) develop ways to control transgene integration, or 4) develop ways to expand a small number of genetically characterized modified stem cells in vitro and then re-infuse these non-oncogenic cells into the patient (Porteus 2006)
These ideas all involve developing more refined methods of designing and administering the treatment, which, realistically, should be created before the therapy becomes approved by the FDA (U.S. Food & Drug Administration 2017).
In addition to passing along side effects or potential errors in germ-line therapy, there is a concern that this technology will be used to genetically enhance future populations. Scientists have theorized the potential to artificially insert genes that correspond to “beneficial” characteristics, such as increased intelligence, height, eye color, et cetera (Diehl 2017). This theoretical scenario becomes the basis for many arguments against the further development of gene therapy. In fact, scholars use a slippery slope argument to bolster their opinion (McGleenan 1995). This argues that if we allow small germ-line or somatic cell therapies now, it will be the first step on a slippery slope towards types of genocide similar to that of the Nazis (McGleenan 1995).
Another concern is that gene therapy could be used to get rid of genetic abnormalities that correspond to certain disabilities. For example, ethicists question whether it would be ethically acceptable to use gene therapy to correct deafness (Cokley 2017). If so, there would have to be some guidelines for what constitutes a genetic abnormality worth correcting (Wirth 2013). However, some believe the act of using gene therapy to get rid of certain disabilities is an act comparable to genocide. Washington Post journalist, Rebecca Cokley details this argument in her article Please don’t edit me out, writing that “nearly 1 out of every 5 people in this country [the U.S.] has a disability. What would it mean for society to render such a large group of people “unfit” for the human germline?” Cokley refers to the notion of editing out disabilities as ableism and argues that disabilities add a richness and diversity to U.S. culture.
Lastly, there are also questions of financial accessibility. If gene therapy was approved and implemented in the near future, treatment would be very expensive (Wirth 2013). There are also questions about whether the treatment would be covered by insurance since it is so experimental. If it is not covered, only the wealthiest individuals would have gene therapy as a therapeutic option. Not only would this create inequity, it could also widen health disparities in the future (Smith et al. 2016). With only the wealthy subset of the population accessing gene therapy, their germline would theoretically get healthier and stronger while those who could not afford gene therapy would lag behind (Smith et al. 2016). Questions get more theoretical when ethicists discuss manipulation of physical characteristics. What would happen to our nation if all the wealthiest individuals were able to pay to have an “intelligence gene” inserted into their embryos? While these questions are all hypothetical at this point, they are important to consider when moving forward with gene therapy research. Gene therapy has great benefits, but it also has the potential to create great inequality and, possibly, take the form of eugenics. Currently, there is not a unified or consistent opinion in the scientific community on how gene therapy should be further developed. Perhaps, the scientific community should come to a consensus on the role of gene therapy before further investigating its potential.
Politics of Health
Issues regarding informed consent are central to the Jesse Gelsinger case as well as recruiting candidates for future clinical trials on gene therapy. Upon further investigation, there was an information gap between researchers and the Gelsingers; they were led to believe the trial was safe when two monkeys had, in fact, died in pre-trial animal studies (Obasogie 2009). Interestingly, this information appeared on consent forms given to the National Institute of Health, but the same statistics were not present on the forms the Gelsingers signed (Obasogie 2009). Additionally, the lead researcher did not disclose that he was running the study with a private company that he had a stake in (Obasogie 2009). Knowing these facts in the wake of Jesse’s death shows the danger of scholarly and financial pursuit at any cost. It also acknowledges the importance of true informed consent. When patients and families are not accurately informed, it can lead them to make decisions that could have irreparable consequences.
This mindset of scientists pushed to fill clinical trials resembles the concept of recruitmentology. Recruitmentology, a term coined by sociologist Steven Epstein, refers to the various means (social, psychological, economic, et cetera) of convincing people to become human subjects in clinical trials (Epstein 2008). While Epstein’s definition was focused on underrepresented groups, the term recruitmentology can also be applied to the Jesse Gelsinger case as well as how scientists are currently recruiting for future trials (Epstein 2008). For example, Gelsinger was convinced by the promise of helping hundreds of other OTCD patients (Obasogie 2009). If the doctors had not emphasized Jesse’s social altruism if he was to enroll, would he still have taken part in the study? It should be important for doctors to understand and manage how they recruit future research subjects. They should ask in an ethical way and not make promises they cannot keep. Additionally, doctors with financial ties to their trial truly should not be able to recruit subjects either. Their financial interests could weigh too heavily on their mind, leading them to twist the truth or leave out important facts so that patients continue to enroll. The comic depicted below shows the skepticism of the public in regards to current physicians’ attitudes towards promoting gene therapy.

Fig. 2 – Cartoon depicting idea of recruitmentology in gene therapy
Gene therapy also has the potential to intersect with the concept of biopower. Biopower, according to Michael Foucault, refers to “forms of power that aim to control, regulate, and monitor a population’s biologic life” (Fürher 2015). Concerns from ethicists, sociologists, and common civilians reflect the idea that gene therapy could take the form of eugenics (Reindal 2000). If gene therapy was recommended to people with chromosomal abnormalities, this manipulation of people’s biology at the most basic level would become an example of biopower. At the most extreme level where gene therapy could be used to create a race of people with “the most desirable” physical characteristics (think specific height, eye color, skin color – whatever they may be), it would be a blatant exertion of power towards controlling a population’s biology. Of course, these possibilities are theoretical since the science has not progressed to this extent yet. However, scholars have referenced these examples of biopower when cautioning scientists against future gene therapy research (Reindal 2000). It will be interesting to see how gene therapy progresses in the future. Until then, it is important for scientists to continue considering the ethical implications of manipulating their patients’ genetic makeup.
References
Cepko, Constance L. et al. “Construction and applications of a highly transmissible murine retrovirus shuttle vector.” Cell 27, no. 3 (1984): 1053-1062.
Cokley, Rebecca. “Please don’t edit me out.” The Washington Post. Last Modified August 10, 2017. https://www.washingtonpost.com/opinions/if-we-start-editing-genes-people-like-me-might-not-exist/2017/08/10/e9adf206-7d27-11e7-a669-b400c5c7e1cc_story.html?utm_term=.711c53a6ea9f
Cotrim, Ana P. and Bruce J. Baum. “Gene Therapy: Some History, Applications, Problems, and Prospects.” Toxicologic Pathology 36, no. 1 (2008): 97-103.
DeWeerdt, Sarah. “Gene Therapy: a treatment coming of age.” The Pharmaceutical Journal. Last modified October 6, 2014. http://www.pharmaceutical-journal.com/news-and-analysis/features/gene-therapy-a-treatment-coming-of-age/20066677.article
Diehl, Paul. “Germline Gene Therapy Concerns.” The Balance. Last Modified May 6, 2017. https://www.thebalance.com/what-is-the-concern-over-germ-line-gene-therapy-375621
Epstein, Steven. “The rise of ‘recuitmentology’: clinical research, racial knowledge, and the politics of inclusion and difference.” Social Studies of Science 38, no. 5 (2008): 801-832.
Friedmann, Thomas and R. Roblin. “Gene therapy for human genetic disease?” Science 175, no. 4025 (1972): 949-955.
Führer, Amand. “Statistics and sovereignty: the workings of biopower and epidemoiology.” Global Health Action 8 (2015): 1-2.
“How FDA Evaluates Regulated Products: Drugs.” U.S. Food & Drug Administration. Last Modified November 16, 2017. https://www.fda.gov/AboutFDA/Transparency/Basics/ucm269834.htm
Hunt, Sonia Y. “Controversies in Treatment Approaches: Gene Therapy, IVF, Stem Cells, and Pharmacogenomics.” Nature Education 1, no. 1 (2008): 222.
McGleenan,T. “Human gene therapy and slippery slope arguments.” Journal of Medical Ethics 21, no. 6 (1995): 350-355.
Mestrovic, Tomislav. “What Are Viral Vectors?” Medical News – Life Sciences. Last Modified October 27, 2015. https://www.news-medical.net/life-sciences/What-are-Viral-Vectors.aspx
Obasogie, Osagie K. “Ten Years Later: Jesse Gelsinger’s Death and Human Subjects Protection.” The Hastings Center. Last modified October 22, 2009. http://www.thehastingscenter.org/ten-years-later-jesse-gelsingers-death-and-human-subjects-protection/
“Ornithine transcarbamylase deficiency (OTCD)” National Institutes of Health. Last modified November 14, 2017. https://ghr.nlm.nih.gov/condition/ornithine-transcarbamylase-deficiency
Patil, P.M. et al. “Review Article on Gene Therapy.” International Journal of Genetics 4, no. 1 (2012): 74-79.
Reindal, Solveig M. “Disability, gene therapy and eugenics – a challenge to John Harris.” Journal of Medical Ethics 26 (2000): 89-94.
Rosenberg, Steven A. et al. “Gene Transfer into Humans – Immunotherapy of Patients with Advanced Melanoma, Using Tumor-Infiltrating Lymphocytes Modified by Retroviral Gene Transduction.” The New England Journal of Medicine 323, no. 9 (1990): 570-578.
Smith, Caren E et al. “Using Genetic Technologies to Reduce, Rather Than Widen, Health Disparities.” Health Affairs 35, no. 8 (2016): 1367-1373.
“What is Gene Therapy?” National Institutes of Health. Last modified November 7, 2017. https://ghr.nlm.nih.gov/primer/therapy/genetherapy
Whipple, Tom. “New gene therapy ‘shrinks tumours like ice cubes’ The Times. Last Modified March 1, 2017. https://www.thetimes.co.uk/article/new-gene-therapy-offers-hope-for-cancer-patients-dl2m7zmzs
Wilson, Jennifer F. “Murine Gene Therapy Corrects Symptoms of Sickle Cell Disease.” The Scientist. Last modified March 18, 2002. http://www.the-scientist.com/?articles.view/articleNo/13916/title/Murine-Gene-Therapy-Corrects-Symptoms-of-Sickle-Cell-Disease/
Wilson, Robin F. “The Death of Jesse Gelsinger: New Evidence of the Influence of Money and Prestige in Human Research.” American Journal of Law & Medicine 36, (2010): 295-325.
Wirth, Thomas et al. “History of gene therapy.” Gene 525, no. 2 (2013): 162-169.
« Back to Glossary Index