Research
Research in the Rex Laboratory is split into two main areas of interest.
1. Traumatic injury
An estimated 300,000 service members were exposed to improvised explosive devices in the recent wars in Iraq, resulting in traumatic brain injury (http://veterans.rand.org). Recently, a group of Veterans with mild traumatic brain injury due to blast exposure, who retained closed globes, were shown to have ocular pathologies including corneal abrasions, hyphemas, cataracts, corneal edema, angle recession, hemorrhage, retinal tears or detachments, macular holes, choroidal rupture, commotio retinae, and optic neuropathy (Blanch and Scott, 2009; Cockerham et al., 2011). Unfortunately, most service members and Veterans do not receive this level of examination so the prevalence of vision impairing damage is unknown. Further, since the realization that blast exposure without the presence of perforating or penetrating injury may cause permanent vision loss due to damage to the retina is new, there is a lack of animal models.
One goal of our laboratory is to use our new mouse model of percussive air injury to the eye to study the effects of a primary blast injury to the eye while avoiding confounding complications due to blast exposure to the body of the mouse (such as air emboli). Our model mimics an open field waveform and exposure is limited to the eye of the mouse. We have detected vision loss and damage to the retina, and other ocular structures, using this model. We are now assessing cell death pathways, genetic controls, and biomarkers, and testing therapeutic interventions.
2. Glaucoma and Neuroprotection
Our long-term goal is to treat glaucoma using systemic neuroprotective gene therapy. Nearly 3 million people have been diagnosed with glaucoma, a leading cause of blindness in the United States. Current therapies are directed at lowering the intraocular pressure (IOP), the most significant risk factor for the development of glaucoma. However, the need for daily treatment results in poor patient compliance. We offer an alternative, IOP-independent, neuroprotective therapy, a modified form of erythropoietin (EPO). EPO is already FDA approved for the treatment of anemia. We modified EPO to diminish the erythropoietic activity while preserving its neuroprotective activity, and packaged it into an adeno-associated viral vector (rAAV) to provide sustained, systemic delivery. Transduction of peripheral muscle by a single injection protects 70% of RGC somata and axons, and preserves visual function in the DBA/2J mouse. Our current study tests two conceptual hypotheses:
1. EPO-R76E, blocks astrocyte hypertrophy and the resulting mechanical injury to the axons and secondary RGC death by downregulation of the water channel, AQP4.
2. Synapse targeting by the immune system initiates the cascade of RGC death in glaucoma. We propose that treatment with rAAV.EpoR76E blocks synapse targeting by C1Q, thus preserving normal synaptic connections and visual function.
These hypotheses will be tested in the DBA/2J, and additional novel mouse models of glaucoma.
Confocal micrographs of labeled retinal ganglion cells in flat-mounted retinas from a young, non-glaucomatous DBA/2J mouse; a glaucomatous DBA/2J mouse treated with rAAV.eGFP; and a glaucomatous DBA/2J mouse treated with rAAV.EpoR76E.
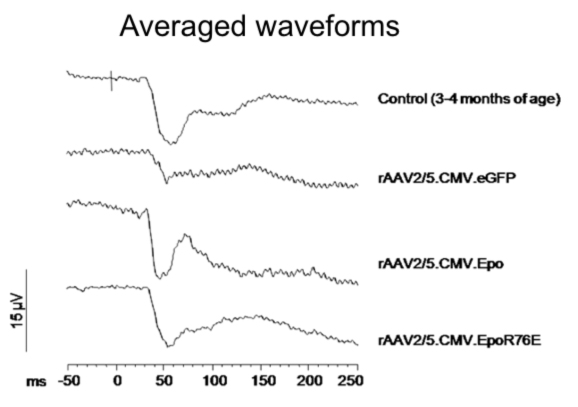
Averaged visual evoked potential waveforms after exposure to a flash of white light. Young DBA/2J mice show the characteristic waveform. Both the N1 and P1 amplitudes are diminished in glaucomatous mice treated with rAAV.eGFP. The amplitude is preserved when the mice were treated with either rAAV.Epo or rAAV.EpoR76E.
Sullivan TA, Geisert EE, Hines-Beard J, Rex TS. (2011) Systemic AAV-mediated gene therapy preserves retinal ganglion cells and visual function in DBA/2J glaucomatous mice. J Hum Gene Ther. 22: 1191-1200.
The lab is supported by Federal Grant Support:
NEI R01 EY022349 Novel Therapy and Mechanisms in Glaucoma
DoD Vision Research Program Award W81XWH-10-1-0528 Treatment of Vision Loss in a New Mouse Model of Blast Injury.

Connect with Vanderbilt
©2025 Vanderbilt University ·
Site Development: University Web Communications