ADDRESSING THE MEDICAL NEED
Diabetes is an increasingly prevalent disease that affects 1 of every 11 Americans today. That’s approximately 29 million Americans. Diabetes can lead to downstream complications like heart disease, nerve damage, and diabetic foot ulcers (DFUs). DFUs, though small at first, have the potential to develop into very costly occurrences. DFUs are categorized based on their severity and can range from superficial redness of the skin (stage 0) to deep wounds that damage underlying nerves, muscles, and bone (stage V). More serious wounds often require surgical intervention, which includes amputation of the foot or leg, and can cost upwards of $45,000 for a non-ideal solution.
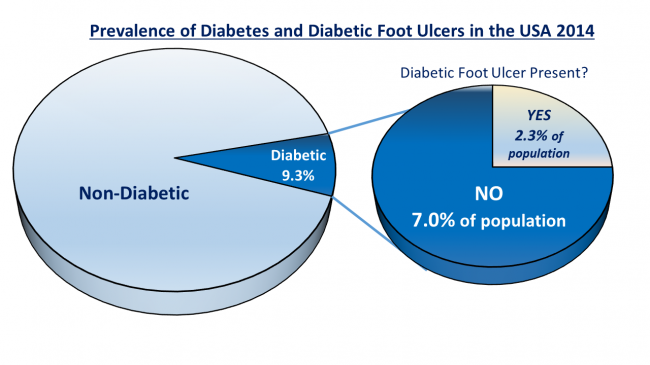
Astonishingly, there is no active healing process or treatment for these wounds. A current standard of care (SoC) involves using a total-contact cast to remove any load or pressure onto the wound. This method isolates the wound to allow it to heal and prevent further damage, but does not actively work to heal the wound. There is a need for a device that directly promotes wound healing, complements current standard and procedures, and maintains patient compliance.
Project Objective
LumaSil seeks to add an active form of healing DFUs onto a SoC. Low-level light therapy has been previously proven to accelerate the rate of shallow-wound healing up to 50% (Whelan). By incorporating this therapy into the full-contact cast used as a SoC, this device has the potential to alter current standard practices. However, in order to do so, this device must meet specific needs regarding the patient, the provider, and the general healthcare system. In addition to the above, the device must be small, easily-portable, waterproof, and automated in order to assure patient compliance and be easily adopted. The provider must find application of the device simple and requiring minimal preparation time. Finally, the product should be low cost, dependable, and durable in order to it to be viable in the medical device industry. These traits should also allow the product to be adopted into various insurance plans, in order to be a practical treatment option.
Whelan, Harry T., Robert L. Smits, Ellen V. Buchman, Noel T. Whelan, Scott G. Turner, David A. Margolis, Vita Cevenini, Helen Stinson, Ron Ignatius, Todd Martin, Joan Cwiklinski, Alan F. Philippi, William R. Graf, Brian Hodgson, Lisa Gould, Mary Kane, Gina Chen, and James Caviness. “Effect of NASA Light-Emitting Diode Irradiation on Wound Healing.” Journal of Clinical Laser Medicine Surgery 19.6 (2001): 305-14

©2026 Vanderbilt University · ©2015 J-BALLS LLC
Site Development: University Web Communications