#4 – January 20, 2016
Posted by schlunsg on Wednesday, January 20, 2016 in Progress Report.
Progress Report #4
Winter break saw great advances in both our IRB application as well as prototype designs for our device, which we have now named LumaSil, which is derived from lumen, the Latin word for “light”, and silicone, which is the main component of our patch. Since our last progress report, the following has occurred:
- Divided the team into two sets of three. The first group will continue to complete the IRB application which we intend to submit by the first week of February. The second group is beginning to finalize the physical design as well as produce a prototype, which is intended to be completed around the same time.
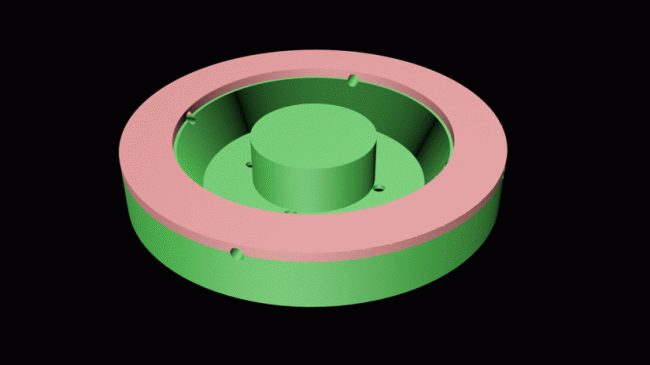
- After our most recent meeting with Dr. Adam Hicks, we have realized the need to streamline the patch into a much more low-profile design. Using 3D modeling software, we have created a new mold design which breaks into three parts, as shown in the following image (clicking the image will demonstrate how it is connected). This mold was printed at the Design Studio and a prototype using a silicone sample was built. This success allows us to proceed, knowing that the design is feasible and efficient.
- With the success of the patch design, we have begun testing and designing a functional circuit for the non-replaceable part of the device. The circuit will contain a set of blue (470 nm) and IR (850 nm) LEDs that will pulsate for a specific duration (10 minutes currently) at a specific frequency every 24 hour period. The light from these LEDs will be carried along the fiberoptic cables to the patch at the site of the wound.
Finally, the IRB part of our group will be meeting with MDRAP next week to confirm some details about the IRB application as well as complete the risk assessment. The next week will primarily be spent advancing the IRB application and completing the design for the circuitry.

©2026 Vanderbilt University · ©2015 J-BALLS LLC
Site Development: University Web Communications