Research Focus Areas
1) Regulation of Migration and Invasion by Exosomes
Migration and invasion are fundamental cell processes that are critical for a diverse physiological and pathological processes, including cancer metastasis, immune function, wound healing and tissue repair, brain development and angiogenesis. Cell migration is a complex process that involves reorganization of the internal actin cytoskeleton in response to external cues and an intricate back-and-forth communication with the extracellular environment. Recently, we and others have found that a major function of exosomes is to promote cellular migration and invasion.
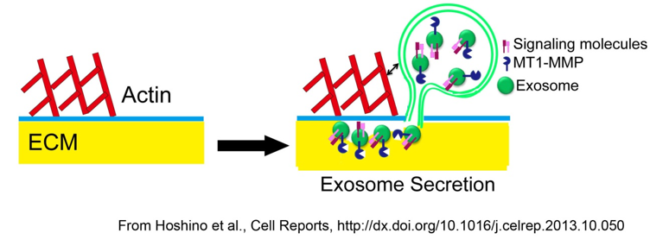
Exosomes are small EVs that are formed within multivesicular endosomes (MVE) within cells and released by docking and fusion of MVE with the plasma membrane (Fig 1). We found that actin-rich invasive structures formed by cancer cells called invadopodia are hotspots for MVE docking and release of exosomes. Furthermore, those exosomes carry proteinases and other molecular that function to promote cellular invasion. We expect that in non-cancer cells similar actin-rich cortical structures may function similarly to enhance exosome secretion.
Figure 1. Model of exosome secretion at invadopodia. Exosome-carrying multivesicular bodies are docked at invadopodia and secrete growth factor and proteinase-containing exosomes that enhance invadopodia stability, activity and lead to additional de novo invadopodia formation.
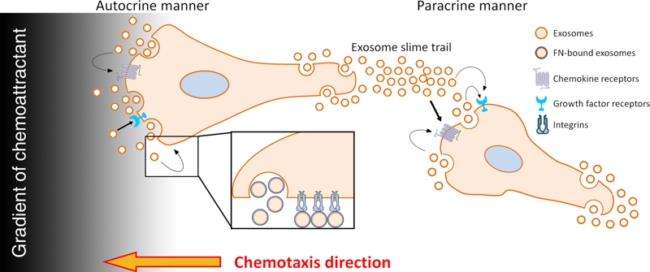
To understand the function of exosomes in cell migration, we carried out a series of genetic inhibition and rescue experiments in cancer cells. We discovered that exosome secretion is essential for directional sensing of cells during migration in in vivo environments and in response to chemotactic gradients (Fig 2). We also found that exosome secretion is important to promote nascent adhesion formation and cell speed. In addition, our live imaging data have revealed that exosomes are left in “slime trails” behind migrating leader cells that can promote pathfinding behavior of follower cells in a paracrine manner.
Figure 2. Model for regulation of migration by exosome secretion. Exosomes deliver both chemotactic factors and extracellular matrix to promote directional migration and adhesion formation. From Sung et al., 2017
2) Imaging tools
A key tool for for the study of cell migration is live imaging. In order to visualize exosome secretion at subcellular sites in migrating cells, we developed a novel reporter for exosome secretion, pHluorin-CD63. CD63 is a tetraspanin membrane protein that is enriched on exosomes and frequently used as a marker of multivesicular endosomes (MVEs). By inserting a pH-sensitive GFP derivative, pHluorin into a small extracellular loop of CD63, MVB fusion and exosome secretion can be imaged at subcellular locations in real time. We further recently developed a stable mutant form of this protein, pHluorin_M153R-CD63, that can be utilized for stable expression in cells with brighter and higher resolution.
Related publications are:
Hoshino, D, Kirkbride, KC, Costello, K, Clark, ES, Sinha, S, Grega-Larson, N, Tyska, MJ, and Weaver, AM, “Exosome secretion is enhanced by invadopodia and drives invasive behavior”, Cell Reports, 5:1159-1168, 2013. PMCID: PMC3873329
Sung, BH, Ketova, T, Hoshino, D, Zijlstra, A, and AM Weaver, “Directional cell movement through tissues is controlled by exosome secretion”, Nature Communications, 6:7164, 2015. *Recommended by Faculty of 1000. PMCID: PMC4435734
Sung, BH and AM Weaver, Exosome secretion promotes chemotaxis of cancer cells”, Cell Adhesion and Migration, 11:187-195, 2017. PMCID: PMC5351719
Jimenez, L, Yu, H, McKenzie, AJ, Franklin, JL, Patton, JG, Liu, Q, and AM Weaver, “Quantitative proteomic analysis of small and large extracellular vesicles (EVs) reveals enrichment of adhesion proteins in small EVs”, J. Proteome Res., 18:947–959, 2019. PMCID: PMC6411421
Sung, BH, von Lersner, A, Guerrero, J, Inman, D, Pelletier, R, Zijlstra, A, Ponik, S.M., and AM Weaver, “pHluo_M153R-CD63, a bright, versatile live cell reporter of exosome secretion and uptake, reveals pathfinding behavior of migrating cells.”, Nature Communications, 11:2092, 2020 PMCID: PMC7190671
3) Trafficking of RNAs into EVs
Exosomes and other EVs carry not only protein but also RNA cargoes, including miRNAs and other noncoding RNAs. Delivery of RNAs in exosomes can alter gene expression in the tumor microenvironment and potentially induce transformation; however more characterization needs to be performed to define which RNAs are enriched in exosomes and whether they are functional. A key to understanding this process is identification of RNA scaffolding and trafficking routes in the cell, as well as how they are regulated by cancer cell signaling. We are currently working on how RNAs and RNA-binding proteins are selected for incorporation into EVs, using colon cancer and mesenchymal stem cells as model systems. We recently received a program project grant from NCI (more information here) in this area.
Related publications are:
Cha, DJ, Franklin, JL, Dou, Y, Liu Q, Higginbotham, JN, Beckler, MD, Weaver, AM, Vickers, K, Prasad, N, Levy, S, Zhang, B, Coffey, RJ, and Patton, JG, “KRAS-dependent sorting of miRNA to exosomes”, eLife, 4:e07197, 2015 doi: 10.7554/eLife.07197. PMCID: PMC4510696
McKenzie, AJ, Hoshino, D, Hong, NH, Cha, DJ, Franklin, JL, Coffey, RJ, Patton, JG, and Weaver, AM, “KRAS-MEK signaling controls Ago2 sorting into exosomes”, Cell Reports, 15:978-987, 2016. PMCID: PMC4857875
Hinger SA, Cha DJ, Franklin JL, Higginbotham JN, Dou Y, Ping J, Shu L, Prasad N, Levy S, Zhang B, Liu Q, Weaver AM, Coffey RJ, Patton JG, “Diverse Long RNAs are differentially sorted into extracellular vesicles secreted by colorectal cancer cells”, Cell Reports, 25:715-725.e4, 2018. PMCID: PMC6248336
Barman, B, Ping, J, Krystofiak, E, Allen, R, Prasad, N, Vickers, K, Patton, JG, Liu, Q, and Weaver, AM, “Biogenesis of RNA-containing extracellular vesicles at endoplasmic reticulum membrane contact sites.” https://www.biorxiv.org/content/10.1101/2020.12.04.412379v1
4) Role of tumor cell EVs in cancer aggressiveness and metastasis
EVs are a major way that cells in the tumor microenvironment communicate. EVs have been shown to promote fibroblast activation, angiogenesis, and immune suppression. In addition, EVs have been shown to seed pre-metastatic niches, facilitating tumor cell colonization at distant sites. We are actively interested in understanding all of the ways that cancer-derived EVs drive cancer aggressiveness and metastasis and have multiple active projects in this area, including in breast cancer, lung cancer, colon cancer, and head and neck cancer.
A recent related publication is:
Sato, S, Vasaikar, S, Eskaros, A, Kim, Y, Lewis, JL, Zhang, B, Zijlstra, A, and AM Weaver, “EPHB2 carried on small extracellular vesicles induces tumor angiogenesis via activation of ephrin reverse signaling”, JCI Insight, 4(23):e132447, 2019. https://doi.org/10.1172/jci.insight.132447. PMCID: PMC6962030
5) EVs as cancer biomarkers in liquid biopsies
EVs are present in all body fluids and represent the product of active secretion/release from cells. Thus they represent a natural “snapshot” of health and disease in the body. We are interested in translating our basic science findings by developing EV-based biomarkers to identify patients at risk of cancer metastasis/recurrence or for early detection of undiagnosed cancers. We are currently focusing on small cell lung cancer and head and neck cancers as top priorities in this area.