Projects
Ongoing Projects
1) G6PC and SLC37A4 mutants in Glycogen storage Diseases (Derek Claxton and Jonathan Sheehan in collaboration with Richard O’Brien)
Glucose 6-phosphatase catalyze the metabolic pathway of hydrolyzing glucose-6-phosphate (G6P) to glucose and inorganic phosphate. Literature survey suggests that insulin and other hormones and metabolites regulate glucose-6-phosphatase gene expression. Increased enzyme activity contributes to the increased Hepatic Glucose Production associated with diabetes. Glucose-6-phosphatase exists as a multicomponent enzyme system composed of a glucose-6-phosphatase catalytic subunit (G6PC), a G6P/Pi antiporter (SLC37A4) and microsomal glucose transporter and a microsomal glucose transporter. The catalytic G6PC exists in three isoforms (G6PC1-3). G6PC1 functionally couples to the SLC37A4 antiporter to hydrolyze G6P. Mutations in G6PC1 that reduce enzyme activity cause Glycogen Storage Disease (GSD) type 1a which is characterized by severe hypoglycemia. This project proposes to obtain a detailed structural and molecular understanding of the mechanisms underlying the catalytic function of G6PC1.
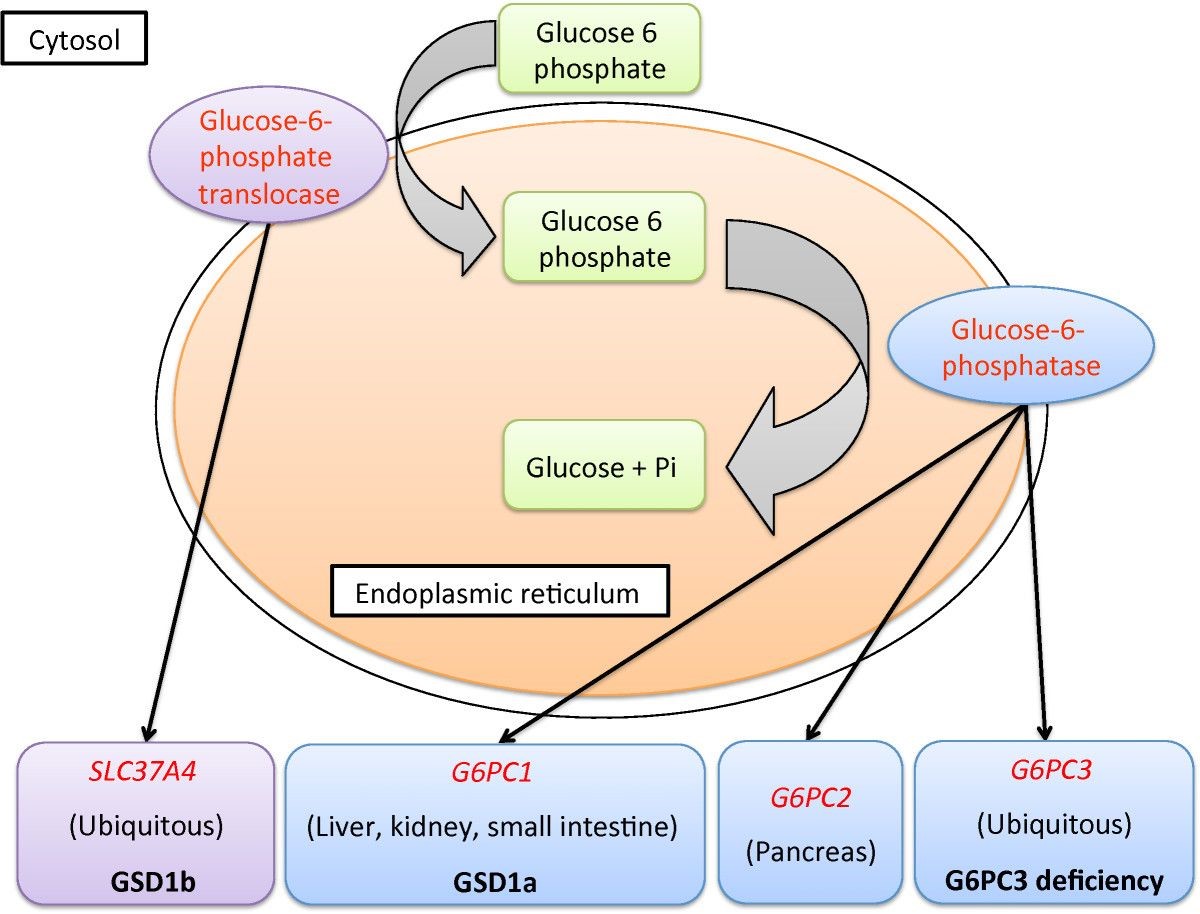
Our goals are to heterologously express and purify G6PC1 while retaining functional activity, to characterize the biophysical activity of the enzyme and do structural analysis through crystallography and cryo-EM.
2) Structure and Mechanism of disease-linked mutants of NMDA receptors (Brinda Selvaraj in collaboration with Erkan Karakas).
NMDAR (N-methyl-D-aspartate Receptors) mediate the majority of excitatory synaptic neurotransmission throughout the central nervous system and are key players in synaptic plasticity, which is important for learning and memory. NMDARs form heterotetrameric assemblies typically composed of two GluN1 and two GluN2 (A-D) subunits. Neurodegenerative disorders, chronic pain, stroke and schizophrenia have all been attributed to the dysfunction of NMDA receptors. Over-activation of NMDA receptors is excitotoxic and contributes to neuronal damage after stroke or traumatic Injury. Furthermore, chronic NMDA receptor hyperactivity gives rise to the loss of neurons associated with Huntington’s, Parkinson’s and Alzheimer’s diseases.
This project is set to analyze the genotypes, functional alterations, and clinical aspects of NMDAR subunit mutations/variants identified from patients with epileptic and schizophrenia. The study will help us to unravel the mystery behind the pathogenicity of the NMDAR mutations and advance our understanding of the subtle and complicated role of the receptors leading to new insights into precision therapy for the neuronal disorders.
3) Mutations in GABA receptor complex from epileptic patients (Jonathan Sheehan in collaboration with Katty (Jing-Qiong) Kang).
GABA is the major inhibitory neurotransmitter in the central nervous system and acts through the GABAA and GABAB receptors. Abnormalities of specific cortical inhibitory neurons and GABA results in anxiety disorders, epilepsy, and neurodevelopmental disorders, including autism. GABAA receptors are ligand-gated Cl− channels responsible for most of the physiological actions of GABA. GABA transporter-1 (GAT-1), encoded by SLC6A1, is one of the major γ-aminobutyric acid transporters in the brain and a major component of GABA signaling.
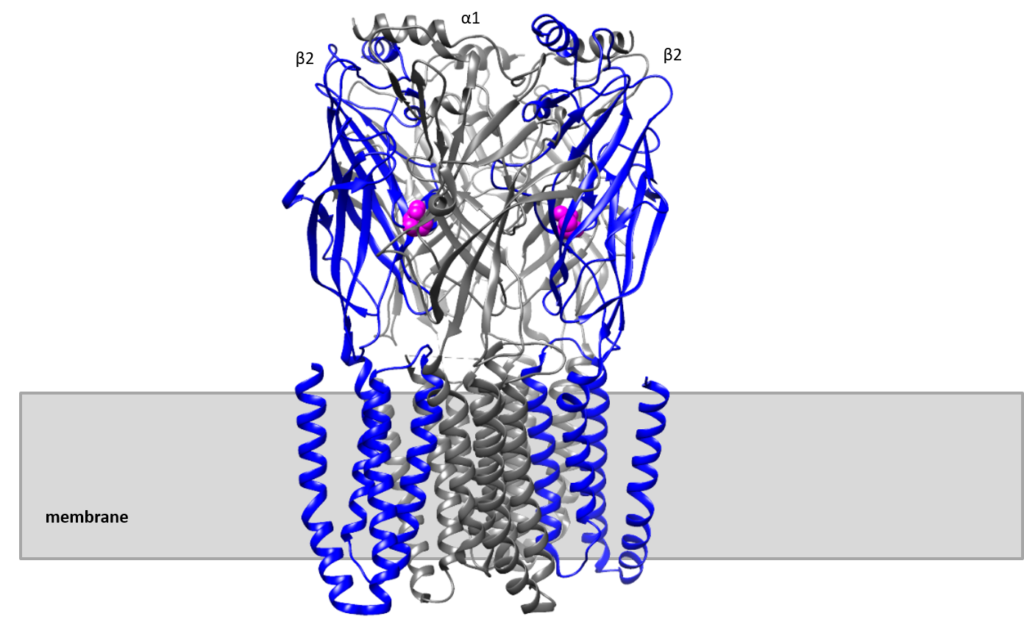
3D reconstruction of GABAA receptor (Hibbs R, et.al., 2018)
We are focused on studying the impact of specific mutants of GABA transporters and receptors on epileptic patients through our computational and experimental expertise.