NSF seed grant supports biomanufacturing of new drug delivery technologies
One of the challenges of drug delivery systems is to optimize their targeting properties so therapeutic compounds used in smaller amounts reach only a specific area of the body and result in little or no side effects.
The ability to engineer the content of extracellular vesicles and target these EVs to specific sites offers the potential to develop a new class of cellular therapeutics that have “exquisite targeting ability” and avoid the side effects and toxicity associated with traditional drug delivery approaches.
EVs are cellular communicators—particles that are naturally secreted and carry a selectively packaged cargo of proteins, nucleic acids, lipids and more from a parent cell to a recipient cell—but do not replicate, unlike cells. EVs are now considered leading actors of intercellular communication, mediating both physiological as well as pathological responses. EV therapeutics are being developed for treatment of a wide range of diseases including diabetes, dementia, heart ailments and cancer.
An interdisciplinary team of Vanderbilt researchers led by Jamey Young, professor of chemical and biomolecular engineering, has received a two-year, $500,000 Future Manufacturing Seed Grant for their work—Enabling Technologies for Biomanufacturing EV-Based Therapeutics—from the U.S. National Science Foundation.
The NSF today announced investments in 24 new projects to drive future manufacturing. The program has awarded more than $40 million in the form of seed grants, research grants and network grants that will support research and education at 44 unique institutions in 18 states and the District of Columbia.
One of 14 NSF seed grants, the Vanderbilt researchers’ long-term goal is to develop technologies for production of “designer EVs” that can be packaged with desired cargo molecules, decorated with tunable surface ligands, and secreted in high yield from specific producer cell types. These technologies for future EV biomanufacturing are expected to promote the growth of a new class of pharmaceutical products.
“Our investment provides industry with manufacturing tools that currently live only in the laboratory, or the imagination,” said NSF Director Sethuraman Panchanathan. “Through the convergence of such fields as robotics, artificial intelligence, biotechnology, and materials research, future manufacturing will create revolutionary products with unprecedented capabilities, produced sustainably in facilities across the country by a diverse, newly trained workforce.”
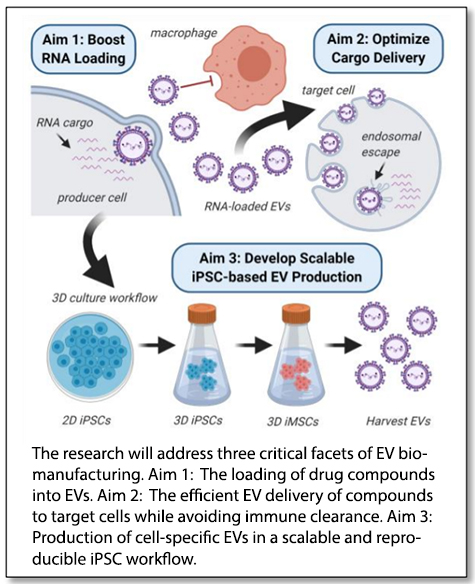 “We will focus our research on loading and delivery of small RNAs that have shown tremendous promise for modulating previously ‘undruggable’ targets but are not efficiently delivered to recipient cells using established nanoparticle-based carriers,” Young said. Senior team members include Ethan Lippmann and John Wilson, assistant professors of chemical and biomolecular engineering, and physician-scientist Alissa Weaver, Cornelius Vanderbilt Professor of Cell and Developmental Biology in the School of Medicine.
“We will focus our research on loading and delivery of small RNAs that have shown tremendous promise for modulating previously ‘undruggable’ targets but are not efficiently delivered to recipient cells using established nanoparticle-based carriers,” Young said. Senior team members include Ethan Lippmann and John Wilson, assistant professors of chemical and biomolecular engineering, and physician-scientist Alissa Weaver, Cornelius Vanderbilt Professor of Cell and Developmental Biology in the School of Medicine.
Ribonucleic acid, or RNA, is a nucleic acid present in all living cells and acts as a messenger carrying instructions from DNA for controlling the synthesis of proteins. Small RNAs are short RNA molecules that can regulate gene expression. Increasing evidence indicates that small RNAs are involved in the development of diverse diseases including cancer, cardiovascular disease, stroke, neurodegenerative disease, diabetes, liver disease, kidney disease and infectious disease. Multiple studies have shown that small RNAs may serve as novel biomarkers and therapeutic targets for many diseases.
“RNA and many other biologic therapeutics, like enzymes, proteins, and DNA, have immense and still almost entirely untapped potential, but critical physiological barriers prevent these molecules from reaching the correct cell type at high enough amounts while sparing healthy tissue,” Wilson said.
Read the full article HERE
