Research Areas
1.) NOVEL OPTICAL NANOTWEEZERS FOR BIOMEDICAL SCIENCE
(Funding: NIH MIRA R35 Outstanding Investigator Award & NSF Electronics, Photonics & Magnetic Devices, EPMD program)
One-half of the 2018 Nobel Prize in Physics was awarded in recognition of optical tweezers. Optical tweezers use a tightly focused laser beam to trap biological objects and have emerged as a powerful tool in biological research, providing the means to non-invasively manipulate microscopic objects such as biological cells. However, the diffraction limit precludes the ability to focus light to the nanoscale to stably trap nanoscale biological molecules such as nanoscale exosomes, proteins, and DNA. Substantially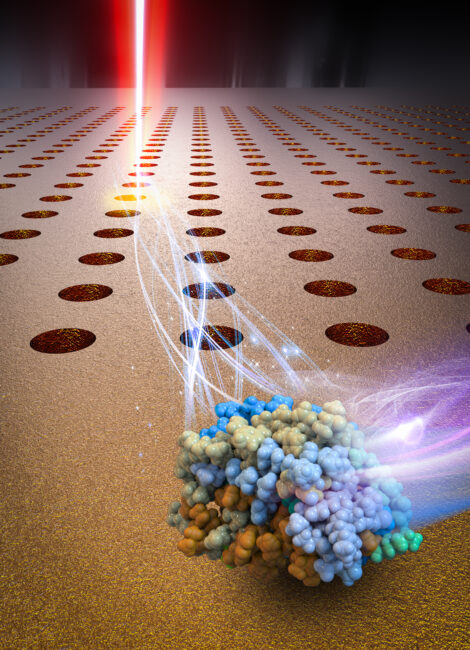 increasing the laser power could increase the trapping stability, but the substantial optical intensity required results in photo-induced damage to the nanoscale biological specimens. Nevertheless, there is a need for novel optical nanotweezers that will enable us to trap and manipulate nanoscale biological molecules too small to be handled using diffraction-limited laser tweezers. This is key to addressing ongoing questions in biology and improving our understanding biological molecules on sub-cellular scales.
increasing the laser power could increase the trapping stability, but the substantial optical intensity required results in photo-induced damage to the nanoscale biological specimens. Nevertheless, there is a need for novel optical nanotweezers that will enable us to trap and manipulate nanoscale biological molecules too small to be handled using diffraction-limited laser tweezers. This is key to addressing ongoing questions in biology and improving our understanding biological molecules on sub-cellular scales.
Our goal: To address this challenge we are developing novel optical nanotweezers that can ‘gently’ handle biological molecules. We have recently invented new optical nanotweezers for trapping and analyzing single nanoscale biological molecules including those in the sub-100 nm and sub-10 nm size regimes. They include: (i) opto-thermo-electrohydrodynamic tweezers (OTET) that feature the trapping and manipulation of sub-10 nm biological objects at a distance far away from the
high-intensity laser focus, where they experience negligible photothermal heating and light intensity; (ii) Geometry-induced Electrohydrodynamic Tweezers that is the most scalable platform for plasmon-enhanced optical trapping capable to placing hundreds or thousands of single nanoscale objects to plasmonic cavities in parallel within seconds; (iii) Anapole enhanced nano-optical tweezers that employed optical anapoles to condense light to the nanoscale for stable trapping of single vesicles or particles without any heating effect. These breakthrough accomplishments have been published in leading peer-reviewed journals including Nature Nanotechnology, Nature Communications, Nano Letters, and also featured by several media outlets including: Nature Research Highlights, The Independent, UK, Futurism, PhysicsWorld, and many others.
References:
- Chuchuan Hong, Justus C. Ndukaife, “Scalable trapping of single nanosized extracellular vesicles using plasmonics”, Nature Communications, accepted (2023), 8 pages.
- I. Hong, C. Hong, Oleg Tutanov, Clark Massick, Mark Castleberry, Qin Zhang, Dennis K. Jeppesen, James N. Higginbotham, Jeffrey L. Franklin, Kasey Vickers, Robert J. Coffey, Justus C. Ndukaife, “Anapole-assisted low-power optical trapping of nanoscale extracellular vesicles and particles”, Nano Letters, accepted (2023), 8 pages
- Sen Yang, Justus C. Ndukaife, “Optofluidic transport and assembly of nanoparticles using an all dielectric quasi-BIC metasurface”, Light: Science and Applications, (2023), 12, 188, 11 pages.
- Chuchuan Hong, Justus C. Ndukaife, “Exosomes trapping, manipulation and size-based separation using opto-thermo-electrohydrodynamic tweezers”, Nanoscale Advances, (2023), 5, pp 2973-2978.
- Theodore Anyika, Chuchuan Hong, Justus C. Ndukaife, “High-speed nanoscale optical trapping with plasmonic double nanohole aperture”, Nanoscale, (2023), 15, pp 9710-9717.
- Sen Yang, Chuchuan Hong, Justus C. Ndukaife, “Nanoparticle trapping in a quasi-BIC system”, ACS Photonics (2021) (https://doi.org/10.1021/acsphotonics.0c01941).
- Chuchuan Hong, Sen Yang, Justus C. Ndukaife, “Stand-off trapping and manipulation of sub-10 nm objects and biomolecules using opto-thermo-electrohydrodynamic tweezers”, Nature Nanotechnology (2020) DOI: 10.1038/s41565-020-0760-z.
- https://www.nature.com/articles/s41565-020-0760-z
Nature Research Highlight : These ‘tweezers’ made of light gently grasp and move a single protein - Justus C. Ndukaife, Yi Xuan, A. G. Agwu Nnanna, Alexander V. Kildishev, Vladimir M. Shalaev, Steven T. Wereley, Alexandra Boltasseva, “High-Resolution Large-ensemble Nanoparticles Trapping with Multifunctional Thermoplasmonic Nanohole Metasurface” ACS Nano (2018)
- Ndukaife, Justus C., Vladimir M. Shalaev, and Alexandra Boltasseva. “Plasmonics—turning loss into gain.” Science 351.6271 (2016): 334-335.
- Ndukaife, Justus C., et al. “Long-range and rapid transport of individual nano-objects by a hybrid electrothermoplasmonic nanotweezer.” Nature Nanotechnology 11.1 (2016): 53.
- Ndukaife, Justus C., et al. “Photothermal heating enabled by plasmonic nanostructures for electrokinetic manipulation and sorting of particles.” ACS Nano 8.9 (2014): 9035-9043.
2.) EXTRACELLULAR VESICLES ANALYSIS
(Funding: NIH MIRA R35 Outstanding Investigator Award, NSF CAREER Award)
Next-generation optical nanotweezers for single nanosized extracellular vesicles analysis: Extracellular vesicles and particles have emerged as a means for cells to communicate with neighboring or distant cells and have generated significant research interests. They contain important biological information molecules such as proteins, DNA, lipids, and miRNA that modify the physiological states of the recipient cells and have been implicated to play a role in the pathogenesis of diverse diseases such as cancer, Alzheimer’s, and Parkinson’s diseases. Due to their small sizes (15 nm to approx. 100 nm), the isolation and analysis of nanoscale extracellular vesicles and particles (EVPs) have been met with challenges.
One of the most significant urgent challenges to overcome in EVP research is understanding the heterogeneity of EVPs. EVPs are heterogeneous in their size and molecular cargo contents. As a result, single EVP analysis has been identified as crucial to deciphering the heterogeneity of individual EVPs and understanding their biological roles in diverse diseases. As an example, an ongoing scientific question concerns whether the newly discovered extracellular particles called exomeres and supermeres are monolithic nanoparticles enriched with multiple makers such as proteins, RNA and lipids or if they are a distribution of different functionally-active nanoparticles (such as proteins, nucleic acid and lipids) co-isolated together. The widely used analysis techniques such as mass spectrometry are incapable of analyzing individual EVPs and hence these assays mask the impact of the heterogeneity of EVPs, which has made it impossible to address this question and other open questions to date.
Our goal: To push the frontier of knowledge in the field, we take an unconventional interdisciplinary approach to tackling biomedical science questions in EVP research. Specifically, we develop new optical nanotweezers enabled by nanophotonics and optofluidics for handling and studying the biochemical properties of nanosized extracellular vesicles as well as their molecular cargo contents to understand their functional roles in diseases and therapeutics. findings from this research program promises to unravel the heterogeneity of EVPs, address open questions, improve our understanding of their functional roles and chart the part for their use in advanced therapeutics
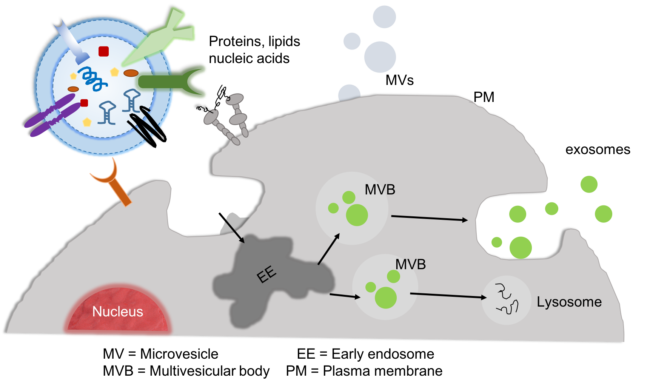
Nanophotonic biosensors for EV liquid biopsy and point-of-care diagnostics:
Studies have shown that cancer cell-derived EVs carry oncogenic macromolecules to normal cells altering their behavior in cancer. This is because cancer-associated EVs carry molecules in their membrane including membrane proteins, lipids, and well-selected DNA, miRNA, mRNA cargo inside them that are derived from the parent tumor cells. EVs are also very abundant in most biofluids, highly stable, and could be analyzed in small volumes (100 μL) of frozen serum or plasma samples, and thus has great potential as a new source of biomarkers for personalized medical diagnosis.
Our goal: We are developing novel plasmonic nanostructures and resonant dielectric nanostructures integrated with microfluidic channels for highly sensitive and specific detection of tumor-associated EVs.
References:
- Chuchuan Hong, Ikjun Hong, Yuxi Jiang, Justus C. Ndukaife, “Plasmonic dielectric antennas for hybrid optical nanotweezing and optothermoelectric manipulation of single nanosized extracellular vesicles”, Advanced Optical Materials, 2024 (Accepted).
- Chuchuan Hong, Ikjun Hong, Sen Yang, Justus C. Ndukaife, “Towards rapid extracellular vesicles colorimetric detection using optofluidics-enhanced color-changing optical metasurface”, Optics Express, 2024 (Accepted).
- Theodore Anyika, Ikjun Hong, Justus C. Ndukaife, “Mirror-enhanced plasmonic nanoaperture for ultrahigh optical force generation with minimal heat generation”, Nano Letters, (2023), 23 (24), pp 11416-11423.
- Ikjun Hong, Theodore Anyika, Chuchuan Hong, Sen Yang, Justus C. Ndukaife, “Hybrid optical and diffusiophoretic nanomanipulation using all-dielectric anapole-enhanced thermonanophotonics”, ACS Photonics (2023), 10 (11), pp 4038-4044. Selected as Cover Art
- Chuchuan Hong, Justus C. Ndukaife, “Scalable trapping of single nanosized extracellular vesicles using plasmonics”, Nature Communications, accepted (2023), 8 pages.
- I. Hong, C. Hong, Oleg Tutanov, Clark Massick, Mark Castleberry, Qin Zhang, Dennis K. Jeppesen, James N. Higginbotham, Jeffrey L. Franklin, Kasey Vickers, Robert J. Coffey, Justus C. Ndukaife, “Anapole-assisted low-power optical trapping of nanoscale extracellular vesicles and particles”, Nano Letters, accepted (2023), 8 pages
- Justus C. Ndukaife, Romain Quidant “Roadmap on Optical Nanotweezers”,J. Phys. Photonics (2023).
- Justus C. Ndukaife, “Merging metasurfaces with microfluidics”, [INVITED], Nature Nanotechnology (2022) 17, pp 1042-1043.
- Sen Yang, Mingze He, Chuchuan Hong, Josh Caldwell, Justus C. Ndukaife, “Engineering electromagnetic field distribution and resonance quality factor using slotted quasi-BIC metasurfaces”, Nano Letters (2022) 22 (20), pp 8060–8067.
3.) NEXT GENERATION NANOPHOTONIC TWEEZERS FOR ENVIRONMENTAL SCIENCE & TECHNOLOGY
(Funding: OPTICA Foundation Challenge Prize)
One of the most pressing challenges facing our global society is the contamination of our environment by plastic wastes. Currently, about 20 million metric tons of plastic wastes are discharged in the ocean annually. Alarmingly, if the current rate of plastic contamination continues, it is projected that by 2050, there will be more plastics than fishes in the ocean by weight. To mitigate this growing concern, urgent action is required to identify efficient strategies and implement new policies aimed at reducing plastic contamination.
Furthermore, understanding the properties and potential health impacts of plastic nanomaterials, specifically nanoplastics (1 nm to 100 nm), is crucial. However, analyzing nanoplastics remains a significant challenge due to their small size. Current analytical tools are limited, hindering our comprehensive understanding of nanoplastic exposure and its consequences.
Our Goal: To address this critical need, this research program aims to develop a groundbreaking technology for the rapid characterization of nanoplastics with single-particle resolution. We propose a high throughput plasmonic nanotweezers approach, merging plasmon-nano-optics and microfluidics, to enable stable trapping and enhanced Raman spectroscopy of individual nanoplastic particles.
4.) ENGINEERING THERMAL RADIATION USING BOUND STATES IN THE CONTINUUM
(Funding: 2024 Office of Naval Research (ONR) Young Investigator Program Award)
The development of affordable and efficient infrared light sources holds significant importance for various defense applications, such as free-space communications, infrared beacons, barcodes, non-dispersive infrared (NDIR) sensing, and surface-enhanced infrared absorption spectroscopies for biological and environmental threat detection. Thermal emission is a crucial method for generating electromagnetic radiation. However, controlling its properties presents key challenges due to its incoherent nature. Thermal emitters typically produce radiation with a broad spectrum, as dictated by Planck’s law of radiation. Consequently, there is a demand for achieving narrow-bandwidth, unidirectional thermal emission with high emissivity within specific wavelength ranges. Previous attempts to control thermal emission using strongly coupled material resonances such as phonon polaritons are limited to specific material platforms and frequencies dictated by the optical phonons of the available materials. Engineered thin films known as metasurfaces have become a new paradigm for engineering the flow electromagnetic radiation. However, the use of dielectric metasurface cavities for thermal emission encountered challenges due to the spectral properties of dielectric resonator materials, like silicon, being temperature-dependent, leading to drift in thermal emission. On the other hand, metallic thermal emitters offer stable performance across a wide temperature range, but the inherent Ohmic losses in metals significantly broaden the emission peak, resulting in a quality factor (Q) of approximately 10.
An intriguing concept derived from quantum mechanics, known as Bound States in the Continuum (BIC), has emerged as a powerful means to control electromagnetic radiation and achieve high Q factors in both metallic and dielectric structures.
Our Goal: In this Young Investigator Program (YIP) program we are investigating the physics of BIC to develop wavelength-selective thermal emitters (WS-EMs). To ensure robust performance without temperature-induced spectral drift in thermal emission, we are investigating BICs in metallic structures and leveraging plasmonic BIC to achieve highly directional thermal emission with a record-breaking high Q factor. We aim to achieve quasi-BIC metasurfaces that will be resilient to temperature fluctuations, possess angular selectivity, and achieve near-unity emissivity with a narrow emission line-width.
References:
- Sen Yang, Mingze He, Josh Nordlander, Joshua Caldwell, Justus C. Ndukaife “Single-peak and narrowband mid-infrared thermal emitters driven by mirror-coupled plasmonic quasi-BIC metasurfaces”, Optica, 2024 (Accepted).
5.) NANOPHOTONIC TRAP AND ENHANCE DEVICES FOR QUANTUM PHOTONICS
(Funding: NSF Connections in Quantum Information Science (CQIS) Program)
Nanoengineered single-photon source: Quantum photonics has been identified as the new frontier where the next technological revolution akin to the transistor and IC revolution of the 20th century will take place. Fundamentally, future quantum photonic technologies and applications rely on the generation, detection, and manipulation of single and entangled photons. To achieve enhanced emission of single and entangled photon pairs on-demand, it is necessary to couple the solid-state quantum emitters to an emission enhancing environment that can provide high Purcell factors for enhancing photon emission rate and efficient outcoupling of emitted photons.
Our goal: To develop nanophotonic cavities and methods for scalable integration of quantum emitters to the nanophotonic cavities towards the realization of ultra-bright single-photon and entangled photon sources that can operate under room temperature conditions.
References:
- Samprity Saha, Chuchuan Hong, Dhruv Fomra, Umit Ozgur, Vitaly Avrutin, Justus C. Ndukaife, Nathaniel Kinsey, “On-chip integrated quantum emitter with ‘trap-enhance-guide’: a simulation approach”, Optics Express(2022) 30, pp 48051-48060.
- Chuchuan Hong, Sen Yang, Ivan Kravchenko, Justus C. Ndukaife, “Electrothermoplasmonic Trapping and Dynamic Manipulation of a Single Colloidal Nanodiamond”, Nano Letters (2021), 21, 12, 4921–492