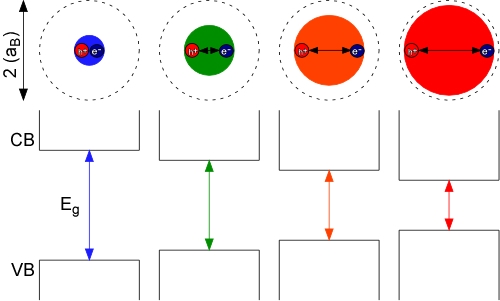
Semiconductor nanocrystals (quantum dots) are single crystals of semiconductor material typically between 1 and 100 nm. Common examples are cadmium selenide, cadmium sulfide, or zinc sulfide nanocrystals. What make these nanocrystals so interesting are their size-dependent optical and electronic properties. These size tunable properties arise from quantum confinement, which is a result of the nanocrystal being smaller than the bulk semiconductor Bohr exciton diameter (2x the Bohr exciton radius, aB). By forcing the electron and hole to occupy a space smaller than the normal equilibrium distance in the bulk material (dotted circles, above), it requires more energy to promote the electron from the valence band to the conduction band; thus, the smaller the nanocrystal, the larger the band gap of the material and the bluer (higher energy/shorter wavelength) the emission from the nanocrystals.
Selected Publications
 Harrison, M. A.; Ng, A.; Hmelo, A. B.; Rosenthal, S. J., CdSSe Nanocrystals with Induced Chemical Composition Gradients. Isr. J. Chem. 2012, 52 (11-12), 1063-1072.
Harrison, M. A.; Ng, A.; Hmelo, A. B.; Rosenthal, S. J., CdSSe Nanocrystals with Induced Chemical Composition Gradients. Isr. J. Chem. 2012, 52 (11-12), 1063-1072.
Niezgoda, J. S.; Harrison, M. A.; McBride, J. R.; Rosenthal, S. J., Novel Synthesis of Chalcopyrite CuxInyS2 Quantum Dots with Tunable Localized Surface Plasmon Resonances. Chem. Mater. 2012, 24 (16), 3294-3298.
McBride, J. R.; Dukes, A. D., III; Schreuder, M. A.; Rosenthal, S. J., On ultrasmall nanocrystals. Chem. Phys. Lett. 2010, 498 (1-3), 1-9.
Rosenthal, S. J.; McBride, J.; Pennycook, S. J.; Feldman, L. C., Synthesis, surface studies, composition and structural characterization of CdSe, core/shell and biologically active nanocrystals. Surf. Sci. Rep. 2007, 62 (4), 111-157.
Swafford, L. A.; Weigand, L. A.; Bowers, M. J., II; McBride, J. R.; Rapaport, J. L.; Watt, T. L.; Dixit, S. K.; Feldman, L. C.; Rosenthal, S. J., Homogeneously Alloyed CdSxSe1-x Nanocrystals: Synthesis, Characterization, and Composition/Size-Dependent Band Gap. J. Am. Chem. Soc. 2006, 128 (37), 12299-12306.
Bowers, M. J., II; McBride, J. R.; Rosenthal, S. J., White-Light Emission from Magic-Sized Cadmium Selenide Nanocrystals. J. Am. Chem. Soc. 2005, 127 (44), 15378-15379.

