Our Research
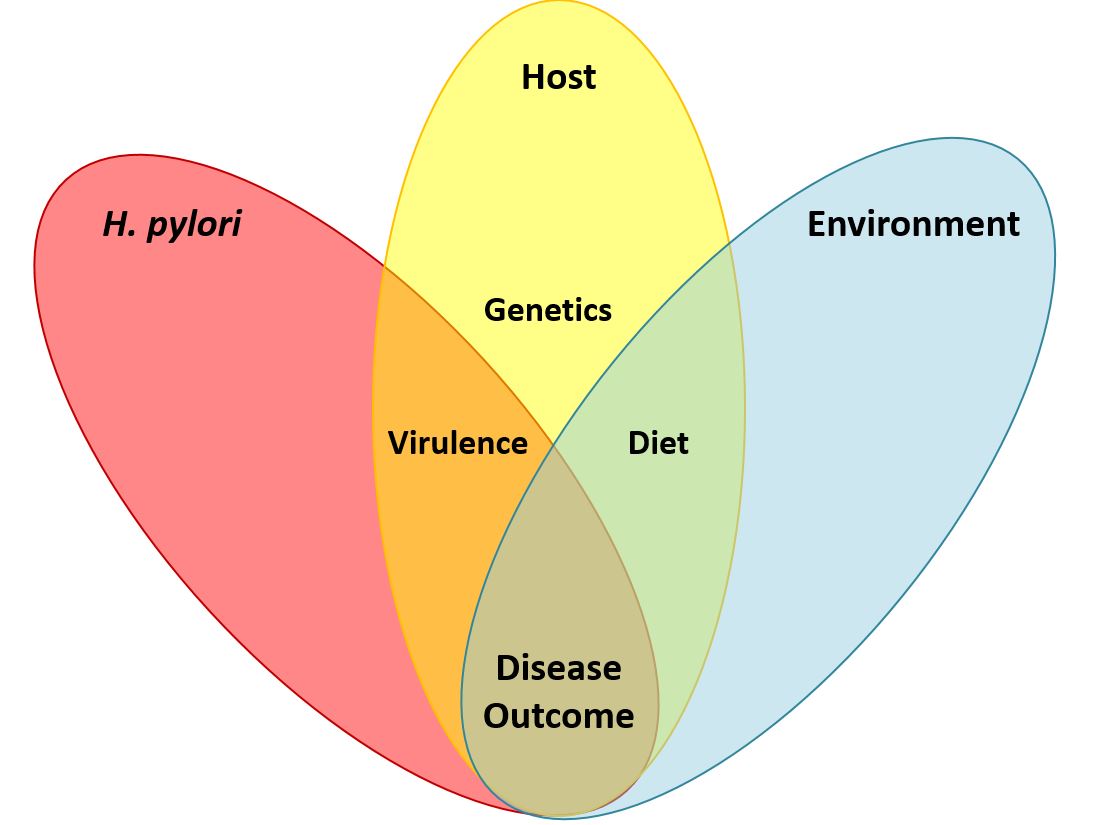
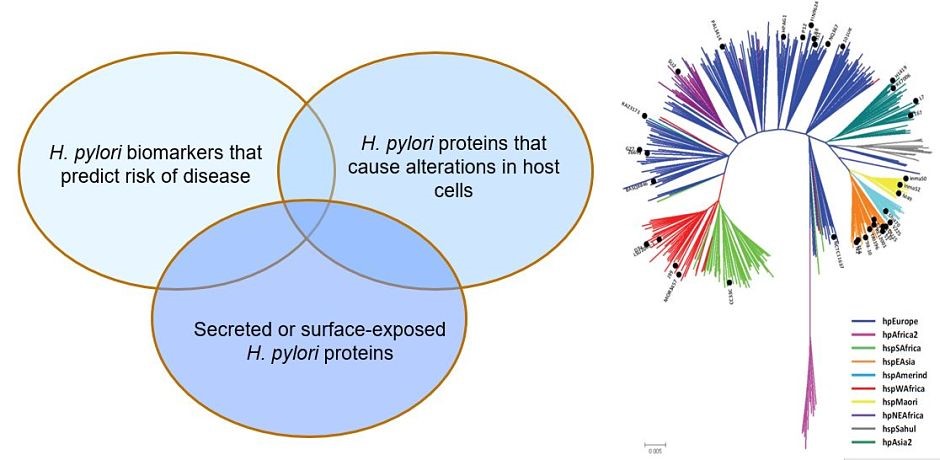
Helicobacter pylori and gastric cancer. Gastric cancer is a leading cause of cancer-related death worldwide, and H. pylori infection is the strongest known risk factor for this malignancy. H. pylori exhibits a high level of intraspecies genetic diversity, and several strain-specific features of H. pylori are linked to development of gastric cancer. These include the presence of a 40-kb chromosomal region known as the cag pathogenicity island (PAI), secretion of active forms of a toxin (VacA), and the production of certain strain-specific outer membrane protein adhesins. We study the activities of these strain-specific factors in cell culture and animal models. We also seek to develop improved methods for identifying H. pylori-infected individuals who have the highest risk of gastric cancer, so they can be targeted for therapeutic intervention.
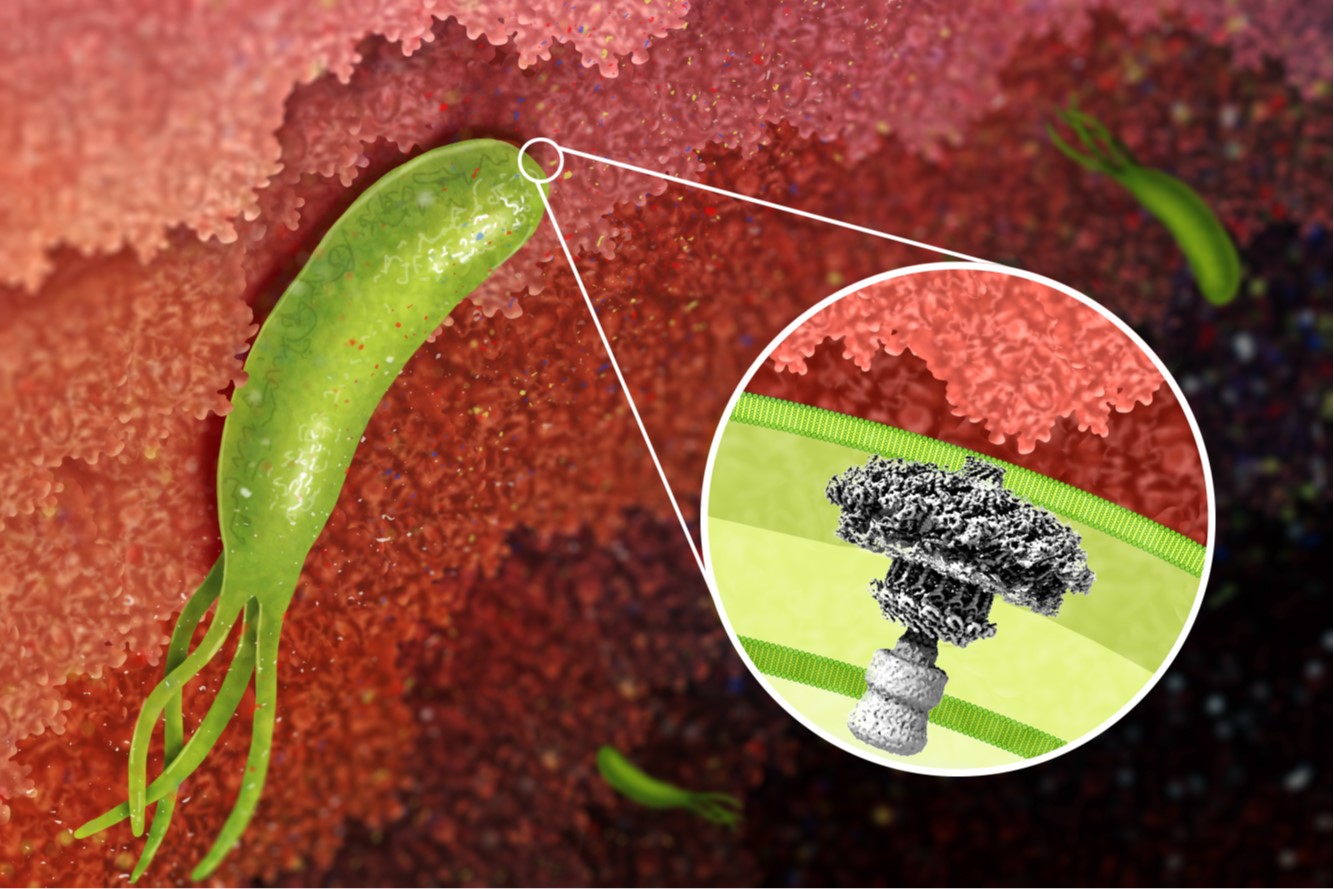
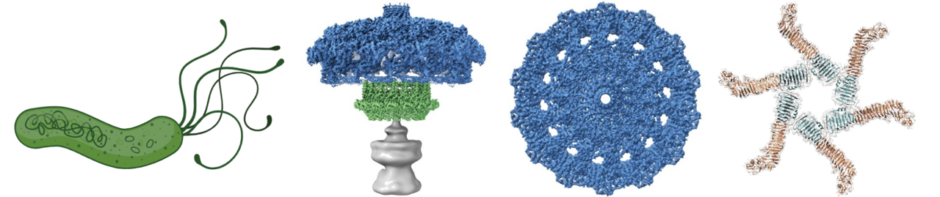
H. pylori cag type IV secretion system. A 40-kb chromosomal region known as the cag pathogenicity island is present in some H. pylori strains but not others. This pathogenicity island encodes an effector protein (CagA) that is delivered into gastric epithelial cells, as well as a type IV secretion system that mediates CagA secretion. CagA causes alterations in host cell signaling and has been designated as a bacterial oncoprotein. We are conducting studies to understand the assembly, molecular architecture, and functional properties of the cag type IV secretion system.
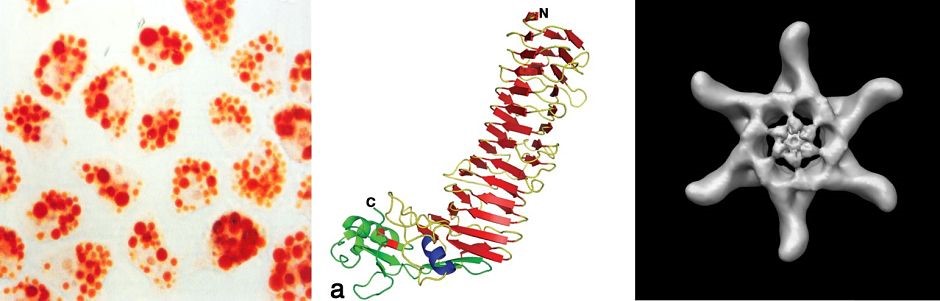
H. pylori VacA toxin. VacA is a secreted channel-forming toxin unrelated to other known bacterial toxins. Most H. pylor istrains contain a vacA gene, but there is marked variation among strains in VacA toxin activity. This variation is attributable to strain-specific variations in VacA amino acid sequences, as well as variations in the levels of VacA transcription and secretion. The most extensively studied VacA activity is its capacity to stimulate intracellular vacuole formation, but the toxin has many additional effects on host cells. Multiple cell types are susceptible to VacA, including gastric epithelial cells, parietal cells, and several types of immune cells. We are conducting studies to understand VacA structure-function relationships and develop a better understanding of the mechanisms by which VacA causes alterations in host cells. In addition, we seek to understand how immunomodulatory actions of VacA influence H. pylori-host interactions in vivo.
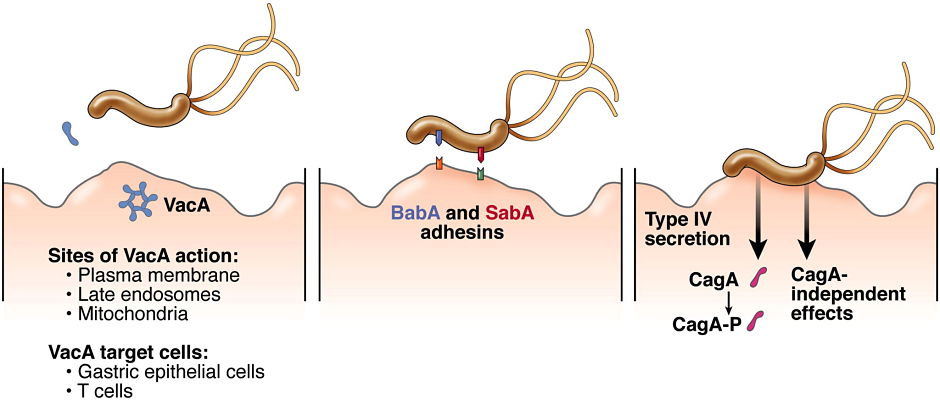
H. pylori outer membrane proteins. H. pylori outer membrane proteins have important roles in bacteria-host cell interactions. We seek to understand the roles of individual outer membrane proteins in bacterial adherence to gastric epithelial cells and colonization of the stomach in animal models.
Dietary composition as a determinant of gastric cancer risk. Epidemiologic studies have shown that a high salt diet is a risk factor for gastric cancer. We are conducting studies to understand how H. pylori gene expression is regulated in response to high salt environmental conditions. In addition, we are analyzing the effects of a high salt diet in animal models of H. pylori infection.
Geographic variation in gastric cancer risk. The incidence of gastric cancer varies markedly throughout the world. Similarly, there is marked geographic variation in genetic features of H. pylori. We hypothesize that the geographic variation in gastric incidence is attributable at least in part to geographic variation in H. pylori virulence. We are conducting comparative genomic studies to analyze H. pylori strains from multiple parts of the world and determine how geographic variation among H. pylori strains may impact gastric cancer risk.