Home » Discovery Grants » Glucose Cycling: A Discovery Grant Project Funding the O’Brien and Young Laboratories (Part I)
Glucose Cycling: A Discovery Grant Project Funding the O’Brien and Young Laboratories (Part I)
Posted by anderc8 on Tuesday, May 30, 2017 in Discovery Grants, News.

Richard O’Brien, Professor of Molecular Physiology and Biophysics
Written by Richard O’Brien, Professor of Molecular Physiology and Biophysics
Two aspects of biological research that are the most rewarding are (i) working on a problem where there is great controversy in the field and (ii) working on a problem where the available data make no logical sense. Our Discovery Grant funds a collaborative research project that is both of these things.
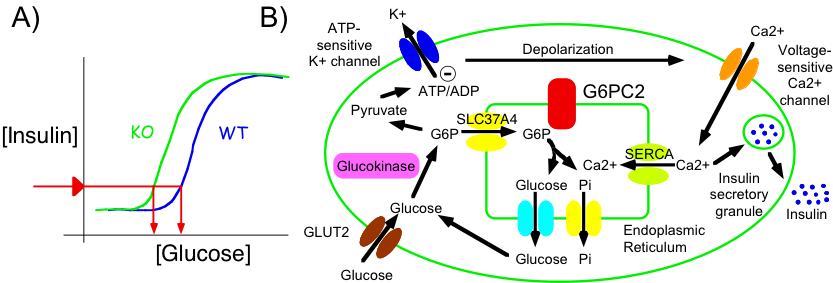
Our project focuses on an intracellular biochemical process called glucose cycling. This process involves two steps. First, glucose is converted into glucose-6-phosphate (G6P) in a reaction catalyzed by an enzyme called hexokinase. Then, G6P is converted back to glucose in a reaction catalyzed by an enzyme called glucose-6-phosphatase. Glucose cycling is an example of a common biochemical phenomenon in which specific metabolites are continuously converted back and forth. These biochemical interconversions are generically referred to as substrate cycles or futile cycles. At face value these cycles seem pointless and inefficient because energy is being wastefully expended during each interconversion. However, it is now appreciated that such apparently futile cycles confer an evolutionary advantage to organisms because flux through biochemical pathways involving futile cycles can be more rapidly modulated and more precisely fine tuned through the control of two opposing enzymes rather than one. For example, if the forward reaction is increased at the same time as the reverse reaction is decreased the net effect is greater. A good analogy would be driving a car – a greater movement forward is achieved if the gas pedal is depressed at the same time as the brake pedal is released.
Experiments studying glucose-6-phosphatase in pancreatic islet beta cells have a controversial history. Multiple groups looked at the level of glucose-6-phosphatase activity in islets and came up with widely varying estimates of how much was there. It’s still not clear why different groups got such different estimates but the modern consensus is that the enzyme is there, but at low levels. Once that was agreed the key question became whether the low level of glucose-6-phosphatase in islets was biologically important. It could have represented some left over event in evolution such that in time, as mammals continued to evolve, glucose-6-phosphatase in islets would be lost entirely.
To address the question as to whether glucose-6-phosphatase in islets was important, different groups measured the amount of glucose cycling. In the 1960s, investigators concluded that, while the enzyme glucose-6-phosphatase was present in pancreatic islet beta cells, glucose cycling did not occur. In the 1990s, investigators looked again using improved methods and concluded that the enzyme glucose-6-phosphatase was present in pancreatic islet beta cells and that glucose cycling did occur, but it occurred at such low rates that it was not biologically important.

Jamey Young, Associate Professor of Chemical and Biomolecular Engineering
This is where the story sat for a while until 1999 when the O’Brien lab at Vanderbilt and John Hutton’s lab at the Barbara Davis Center for Childhood Diabetes in Denver showed that pancreatic islet beta cells contain a special form of glucose-6-phosphatase called G6PC2. The most well studied form of glucose-6-phosphatase called variously G6Pase, G6PC or G6PC1 is highly active in liver cells where it plays an important role generating glucose while we are asleep at night to feed the brain. It had been assumed all along that pancreatic islet beta cells also contained G6PC1 but in fact that turned out to be wrong.
The next breakthrough occurred in 2007 when the O’Brien & Hutton labs showed that mice lacking G6PC2 had altered blood glucose levels. Since G6PC2 is only found in pancreatic islet beta cells this result implied that G6PC2 must be doing something important in pancreatic islet beta cells. But if that was the case then it made no sense that glucose cycling would be low.
Be sure to return to the blog site regularly as my colleague Jamey Young and I will be posting subsequent entries detailing our experiments. For instance, Part II of this blog will be published in the coming days. I also encourage you to join the conversation by leaving comments in the space provided below.