Amritraj (Amrit) Patra
Structural Biology of Poxvirus Protein and Human Antibody Interaction – Single Particle and Computational Analysis of Antibody-Antigen Complexes
Project Abstract
The structure of antibody-antigen complexes will be determined using single particle electron microscopy. The initial focus is to determine structures of neutralizing antibodies to orthopoxviruses bound to antigen by using X-ray crystallography and single particle electron microscopy. Computational approaches will be used to dock high resolution crystal structures of the complexed antibody and antigen into the lower resolution electron density map. The long term goal is to eventually be able to computationally predict the structure of the antibody-antigen complex once the sequence of the antibody is known.
Since some of the antigen-antibody complexes are too small for analysis by single particle EM, Amrit has also started to use X-ray crystallography in his studies.
Project Update
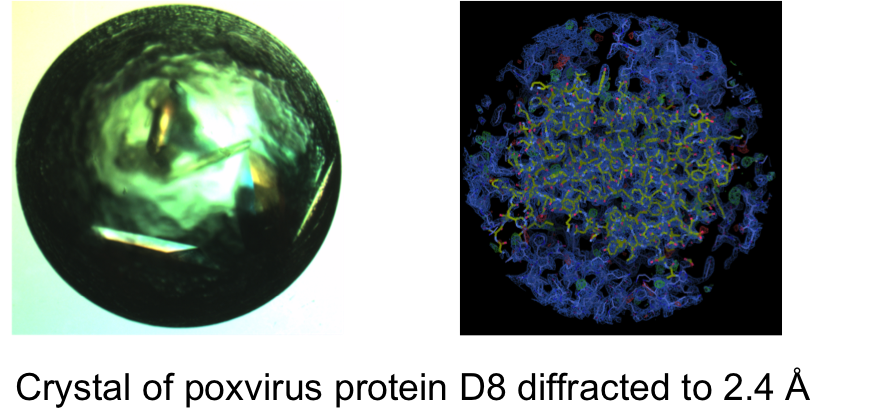
The goal of this project is to recognize the critical interactions an antibody makes to neutralize viruses like orthopoxvirus or norovirus and to computationally redesign human antibodies that can broadly neutralize those viruses. My initial focus is to determine the structures of these antibody-antigen complexes using single particle electron microscopy and x-ray crystallography. Computational approaches will be used to dock high-resolution crystal structures of the non-complexed antibody and antigen into the lower resolution electron density map. The long-term goal is to eventually be able to computationally predict the structure of the antibody-antigen complex once the sequence of the antibody is known.Studies and Results: In the present time period I focused on determining crystal structures of antibody-antigen complexes of poxvirus. The genus Orthopox comprise of four species variola virus (VARV), monkeypox virus (MPXV), cowpox virus (CPXV), and vaccinia virus (VACV). The variola virus is perhaps the most well known because it is responsible for causing smallpox in humans with mortality rates of up to 50%. Fortunately, smallpox was eradicated in 1977. Subsequently vaccination against smallpox was ceased in particular due to rare but severe vaccination adverse events, leaving a large proportion of the current human population vulnerable to orthopoxviruses. The fear that smallpox could potentially re-emerge as a biological weapon and the sporadic outbreaks of zoonotic MPXV and CPXV, has stimulated renewed interest in research on orthopoxvirus protective immunity and treatment. Here at Vanderbilt, Dr. Crowe and colleagues have isolated naturally occurring human monoclonal antibodies from the blood of humans who previously had been infected by or immunized against poxviruses1. Detailed analysis has revealed potent cross-neutralizing human monoclonal antibodies (mAbs) targeting six major orthopoxvirus antigenic proteins namely, H3, A27, D8, L1, B5 and A33. The mAbs cocktail is cross-reactive for VACV, CPXV, MPXV, and VARV and exhibited superiority compared to conventional VIGIV. I used X-ray crystallography to recognize the critical interactions mAbs make with these six antigenic proteins to neutralize the virus. Figure 1. Crystal of poxvirus protein D8 diffracted to 2.4Å (Fig. 1A). Molecular replacement of fourier transform electron density map of the crystal showed the structure of the protein D8 (Fig. 1B). A crystal of antibody-antigen 249Fab/D8 complex was obtained from extended screening of crystallization conditions using liquid-handling robot at the CSB facility (Fig. 1C).VACV protein D8 ectodomain (residues 1-261) was expressed in E. Coli cells and purified by affinity and size exclusion chromatography. Initial crystallization trial of D8 protein was successful with nice looking crystals, which diffracted to 2.4Å (Fig 1). The electron density confirms the presence of D8 protein in the crystal (Fig 1B). With this initial success, a complex of D8 protein and mAb 249 was prepared and crystallization experiments were carried out by sitting drop vapor diffusion in a 96-well format, using the liquid-handling robot at the center for structural biology facility (CSB) at Vanderbilt University. Quality diffracting crystals of D8-249Fab were manually grown at room temperature and are ready to be tested at the APS Synchrotron in Argonne, IL. Literature:1. Gilchuk I. et al.; “Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections”; Cell 2016 167, 684-694.
Publications
Work still in progress
Mentors
Primary: Jim Crowe (formerly Melanie Ohi)
Secondary: Jens Meiler
Type of Trainee
Postdoctoral Fellow
©2024 Vanderbilt University ·
Site Development: University Web Communications