Kevin Kramer
Development of antibody therapeutics for cancer immunology
Project Abstract
Malignancies of the lung are expected to be responsible for over 25% of all cancer-associated mortalities in the United States in 2019. Clinical treatment of lung cancer is complicated by both poor detection of early disease activity and relapse or unresponsiveness to administered therapy. Taken together, these realities of patient outcome underscore the need for alternative therapeutic strategies. Research efforts in the cancer immunology field focus primarily on T cells in the tumor microenvironment, however, there is evidence that B cells may impart a clinical benefit in patients. The formation of tertiary lymphoid structures and antibody secretion in lung tumors associate with positive clinical outcomes yet remain understudied and poorly characterized. In an effort to expand upon these observations, we isolated B cells from cryopreserved human lung cancer tissue and recovered B-cell receptor (BCR) sequences by paired heavy and light chain single-cell RNA sequencing. From these experiments, we identified clonally expanded B-cell populations and convergent BCR sequences shared between different patients. We further demonstrated that recombinant antibodies derived from lung cancer patients bind cultured lung cancer cell lines in a dose-dependent manner. This preliminary data supports the notion that tumor-resident B-cells secrete functional antibodies that may additionally be tumor-reactive. Future characterization of these antibodies includes antigen identification and the measurement of in vitro and in vivo cytotoxic activity. Human patient-derived antibodies not only could serve as a novel source for therapeutic and diagnostic agents but may also help inform vaccine design in lung cancer indications.
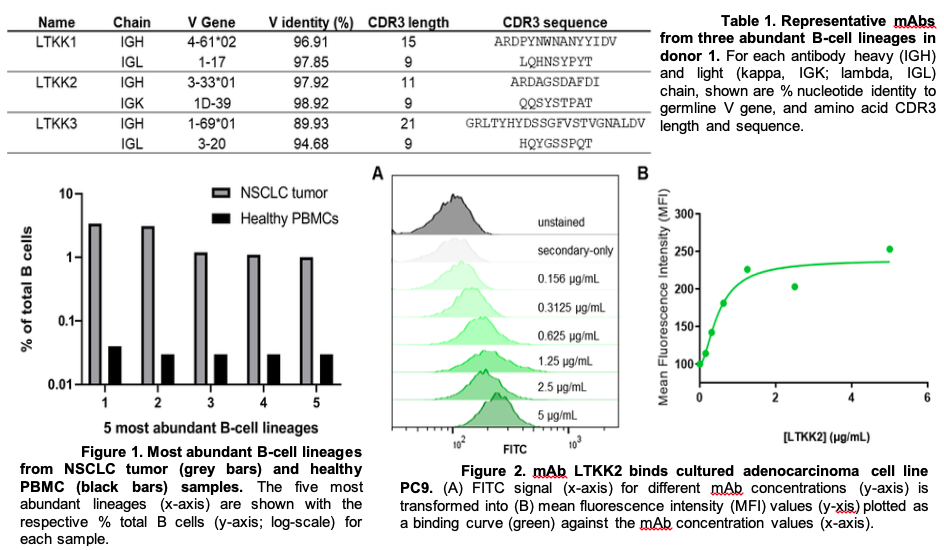
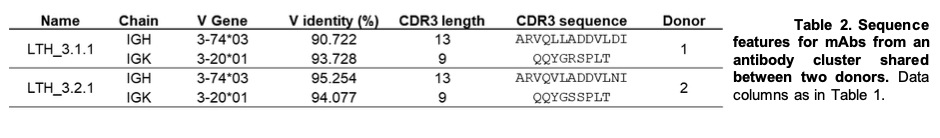
In the past year we made significant progress in the characterization of antibodies from human lung tumor samples. We implemented a novel workflow that included the isolation of B cells from tumor single cell suspensions, preparation of these cells for V(D)J sequencing, and finally functional characterization of abundant sequences as recombinant antibodies. From these efforts we identified abundant B-cell receptor (BCR) sequences within samples and chose a collection to test binding on cultured adenocarcinoma cell lines. We established dose-dependent binding of the candidate antibody LTKK2 to 3 different adenocarcinoma cell lines as well as a bronchial epithelial cell line. We also explored the concept of public antibodies or shared BCR sequences between our patient samples. Indeed, we discovered a number of sequences that shared 70, 80, and 100% amino acid sequence identity in the complementarity determining region 3 (CDR3). Sequence similarity in this structural domain of an antibody sequence could be indicative of a common node of antigenicity shared among patients. Further, we demonstrated one candidate antibody LTH_3.1.1 can bind the adenocarcinoma cell line PC9 in a dose-dependent manner. We are currently developing methods to decipher the antigen specificity of these antibodies. We attempted immunoprecipitation assays, western blots, as well as affinity chromatography columns, but to no avail. Next, we plan on testing these antibody candidates in overlapping peptide as well as antigen microarrays to elucidate their cognate antigen targets. Further characterization of antibodies will include effector function assays as well as in vitro and in vivo efficacy studies. An understanding of the specificity of tumor-infiltrating B cells would help further elucidate a less-characterized department of the tumor microenvironment. Additionally, the public antibody approach could aid in vaccine design from a reverse vaccinology perspective or the analysis of the antibody response to disease rather than immunogen design.
Mentors
Primary: Ivelin Georgiev
Graduate student

©2024 Vanderbilt University ·
Site Development: University Web Communications