Amyn Murji
Development of novel technologies for HIV-1 vaccine design
Abstract
HIV-1 continues to impose a global health burden. Candidate vaccines using HIV-derived antigens have not proven effective to date, and efforts toward protection against new infections remain a high priority in HIV-1 research. In recent years, strategies that target the elicitation of broadly neutralizing antibodies that are capable of neutralizing a large fraction of circulating HIV-1 variants have emerged as a potential avenue to a prophylactic HIV-1 vaccine. The sole target of these neutralizing antibodies is the envelope protein (Env) of HIV-1. However, due to the extensive global diversity of HIV-1, Env-based vaccine candidates so far have only led to the elicitation of antibodies with limited neutralization breadth. To address this challenge, we propose to develop technologies for the presentation of diverse Envs to the immune system. To allow for efficient and accurate evaluation of antibody responses to the designed immunogens, we are developing a binding fingerprinting technology, which applies computational algorithms for predicting the epitope specificities of antibody responses to infection and to vaccination. These novel technologies will be generalizable to vaccine design for other viruses that exhibit high levels of sequence diversity.
Project Update – April 2019
In addition to producing immunogens for the presentation of diverse Envs to the immune system, I developed and characterized epitope-focused immunogens that incorporate conserved regions of Env.
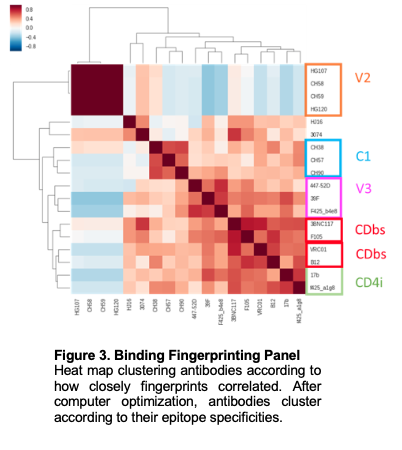
I analyzed immune responses to a 10-week animal study comprised of 4 groups, each with 5 mice. This pilot study sought to compare immunizations with soluble trimer cocktails (with two envs) and nanoparticles displaying both Envs, either as a cocktail with each nanoparticle bearing single env variants, or with each particle bearing both Envs. Preliminary results indicate nanoparticle immunogens perform as well or better than trimers alone. We observed modest heterologous neutralization, as our nanoparticle vaccines elicited serological responses that neutralized an HIV virus not incorporated in our vaccines. Due to these promising results, we completed guinea pig studies with the nanoparticles validated in our mouse animal study and are in the process of further understanding the antibody response elicited to them. An additional 5 HIV-1 Env trimers have been expressed, purified, and characterized via EM in our lab. Subsequently, I have begun expressing each trimer on the ferritin nanoparticle. We are also developing immunogens that incorporate the fusion peptide of HIV Env, a relatively conserved region required for host-cell entry. I have successfully expressed immunogens bearing the fusion peptide of HIV and have expressed and characterized constructs bearing two fusion peptide derivations on ferritin via multicistronic vector technology. Incorporating two different fusion peptides on to ferritin allows us to capture 39.4% of the diversity in that region of globally circulating HIV viruses. Our goals to immunize guinea pigs with these constructs are set to start in a month. Finally, to better understand the response to vaccination, I have assessed the binding of 22 antibodies on 25 antigens and have arrived at a preliminary fingerprinting binding panel that successfully clustered related antibodies together. We soon hope to test our fingerprinting binding panel on guinea pig sera to elucidate binding specificities resulting from nanoparticle immunizations.
Mentors
Primary: Ivelin Georgiev
Secondary: Jim Crowe
Type of Trainee
Graduate Student


©2024 Vanderbilt University ·
Site Development: University Web Communications