Gabriela Alvarado
Molecular determinants of human antibody-mediated inhibition of human norovirus
Project Abstract
The overall goal of this project is to provide a detailed understanding of the human humoral response to Norovirus (NoV) infection and to define the molecular and structural basis for inhibition of NoV by human antibodies. NoV is the leading cause of sporadic and epidemic gastroenteritis in humans. There are currently no vaccines, therapeutics, or prophylactics available to prevent or treat NoV infection. Vaccine design also has been difficult due to the antigenic variation within and between NoV genogroups. To design a vaccine that elicits broad protective immunity, we must have a solid understanding of the NoV-mediated human antibody response to infection and antigenic sites recognized by these antibodies, so a critical area of HuNoV research has become the identification of type specific and cross-genotype and genogroup epitopes. The general hypothesis of this study is that inter- and intragenogroup I and II cross-reactive broadly blocking human antibodies exist and that the blocking function of such antibodies is principally mediated by IgA isotype molecules. The approach to this project will include high-efficiency isolation of human mAbs from patients previously infected with GI and GII strains of human NoV. I then will use this panel of human antibodies to determine if antibody isotype influences binding to NoV virus-like particles (VLPs) and blocking of binding of VLPs to host attachment factors. Finally, using site-directed mutagenesis and three-dimensional structural analysis, I will map immunoreactive epitopes on current circulating strains of NoV and determine whether cross-reactive broadly blocking epitopes exist.
Project Update 2018
As was done below for Norwalk virus, we intend to characterize three-dimensionally where broadly binding and blocking human monoclonal antibodies bind to current circulating strains of norovirus.
Progress Report April 2019
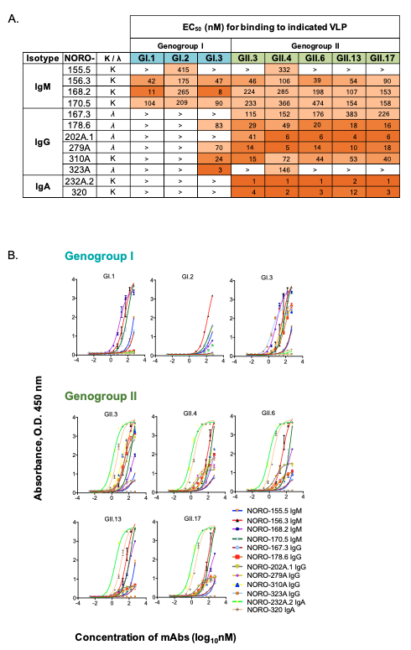
Developing a broadly protective norovirus (NoV) vaccine is challenging due to the antigenic and genetic diversity among circulating strains of human NoV. It is critical to understand the antigenic diversity and identify conserved cross-reactive epitopes on circulating strains of NoV for rational development and testing of new vaccine candidates. To define novel mechanisms of NoV neutralization and to identify antigenic and neutralizing epitopes on circulating strains of human NoV, we isolated and characterized a panel of twelve broadly cross-reactive naturally occurring human IgMs, IgAs or IgGs specific to NoV genogroup I or II (GI or GII) from the memory B cells of immune subjects using human hybridoma technology, as shown in the figure below. Noted are binding EC50s and binding curves for each of the 12 monoclonal antibodies to 8 different norovirus strains, GI.1, GI.2, GI.3, GII.3, GII.4, GII.6, GII.13 and GII.17. Among our panel we noticed three different binding patterns: 1) antibodies that were very broad, binding across GI and GII strains, 2) some that were broad across only GII strains and 3) other mAbs that selectively bound to certain GI and GII strains. Nine of the isolated mAbs also neutralize at least one GI or GII NoV strain when using an in vitro surrogate neutralization system. Binding studies suggested that some of the most cross-reactive mAbs binds to the highly conserved shell domain on the strains tested. X-ray crystallography studies of NORO-320, a GII specific mAb, in complex with GII.4 P domain revealed that NORO-320 does not neutralize by directly inhibiting receptor binding, but instead through steric hindrance. In this study, we isolated a panel of broadly binding and neutralizing anti-NoV human antibodies and identified a novel cross-reactive neutralizing epitope among selected currently circulating GII strains of NoV. These data will be useful when reformulating multivalent VLPs for human NoV vaccine trials, since the goal of a vaccine is to elicit a protective response against diverse circulating strains of human NoV. Some of the human mAbs described here also could be used as biologics for the prevention or treatment of chronic NoV infections or severe NoV disease during outbreaks.
Publications
Alvarado, G. Ettayebi, K., Atmar, R., Bombardi, R., Kose, N., Estes, M., Crowe, J. (2018) Human monoclonal antibodies that neutralize pandemic GII.4 noroviruses. Gastroenterology. 2018 Dec; 155(6):1898-1907
Manzanillo, P., Mouchess, M., Naruhisa, O., Bingbing, D., Ichikawa, R., Wuster, A., Haley, B., Alvarado, G., Kwon, Y., Caothien, R., Roose-Girma, M., Waming, S., McKenzie, B., Keir, M., Scherf, A., Ouyang, W., Tangsheng, Y. (2018) Inflammatory bowel disease susceptibility gene C1ORF106 regulated intestinal epithelial permeability. ImmunoHorizons. 2018; 2(5) 164-171
Mousa, J., Binshtein, E., Human, S., Fong, R., Alvarado, G., Doranz, B., Moore, M., Ohi, M., Crowe, J., (2018) Human Antibody recognition of antigenic site IV on Pneumovirus fusion proteins. PLoS Pathogens. (Accepted)
Mousa, J., Sauer, M., Sevy, A., Finn, J., Bates, J., Alvarado, G., King, H., Loerinc, L., Fong, R., Doranz, B., Correia, B., Kalyuzhniy, O., Wen, X., Jardetzky, T., Schief, W., Ohi, M., Meiler, J., Crowe, J. (2016) Structural basis for nonneutralizing antibody competition at antigenic site II of the respiratory syncytial virus fusion protein. Proceedings of the National Academy of Sciences U.S.A. 2016; 113; E6849-E6858
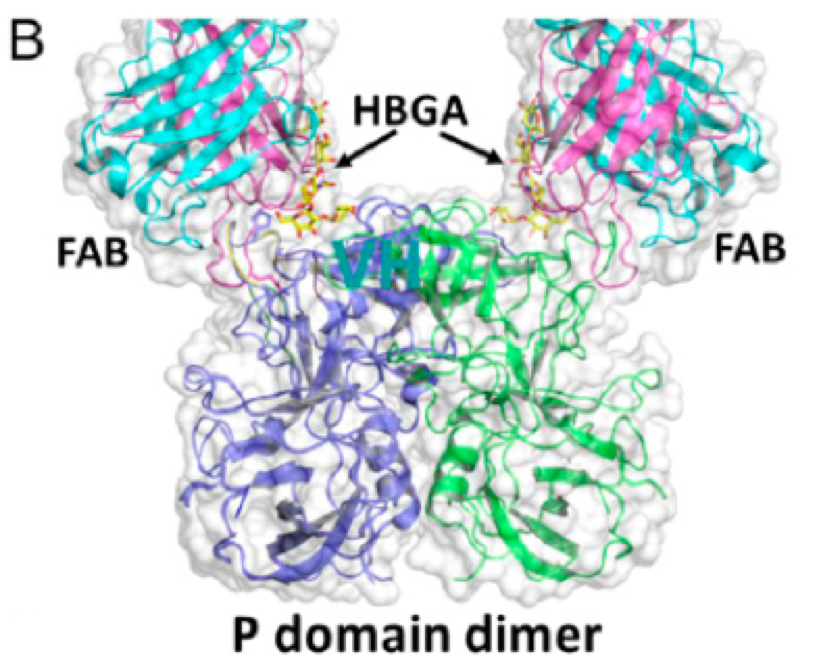
Shanker S, Czakó R, Sapparapu G, Alvarado G, Viskovska M, Sankaran B, Atmar RL, Crowe JE Jr, Estes MK, Prasad BV. Structural basis for norovirus neutralization by an HBGA blocking human IgA antibody. Proc Natl Acad Sci U S A. 2016 Oct 4;113(40):E5830-E5837. PMID: 27647885
Sapparapu, G.*, Czakó, R.*, Alvarado, G.*, Shanker, S., Prasad, B.V.V., Atmar, R., Estes, M., Crowe, J. Jr. (2016). Frequent use of the IgA isotype in human B cells encoding potent norovirus-specific monoclonal antibodies that block HBGA binding. PLoS Pathog. 2016 Jun 29. Doing:10.1371/journal.ppat.1005719. eCollection 2016.
[* co-first authors]
Mentors
Primary: James E. Crowe, Jr.
Secondary: Ivelin Georgiev
Type of Trainee
Graduate student

©2024 Vanderbilt University ·
Site Development: University Web Communications