Marcus Nagel
Structural Studies of Antibody-Antigen Complexes by HDX and Crosslinking Mass Spectrometry
Project Abstract
Hydrogen Deuterium exchange (HDX) and chemical crosslinking (XL) experiments will be carried out to produce structural data and constraints for molecule modeling of antibody-antigen complexes. These chemical approaches will be combined with mass spectrometry (HPLC-MS/MS) analysis to identify specific sites of interaction in these complexes. These data will feed into the pipeline for structural analysis that includes EM, SAS, and crystallographic data in order to model three-dimensional antibody-antigen complexes and to design improved antibodies and vaccines.
Project Update April 2018
Vacuolating cytotoxin A (VacA) – chemical crosslinking (XL) and hydrogen-deuterium exchange (HDX)
To gain further insights into the structure of VacA we performed XL-MS on monomeric VacA (pH 8) and a non-oligomerizing mutant (VacA d346-347). It was possible to identify seven restraints in currently unresolved regions (p33 domain) and three in the resolved p55 domain. These restraints can help, in combination with additional methods like cryo-EM and computational modelling, to create a structural model of the monomeric VacA. Further, these models can be useful to engineer VacA mutants that are less flexible and therefor suitable for x-ray and cryo-EM experiments, without the compromise of the unresolved p33 domain.
Additional experiments were performed for the oligomeric VacA (pH 3) resulting in 23 intermolecular XL-restraints. These restraints can be clustered in three main groups, based on the regions connected and allow the identification the interface between VacA-subunits. Again these can be used to create mutants susceptible to x-ray and cryo-EM analysis, without the missing density of the p33 region or with higher resolution.
Finally HDX-experiments were performed to locate regions involved in oligomerization of VacA, by comparing the wildtype to the mutant d346-347. HDX confirmed one major interaction site, close to the N-terminus.
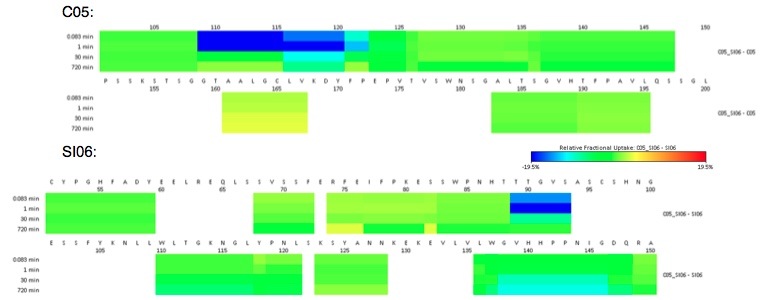
HDX of C05 V110P-A117E (C05) with the head domain of A/Solomon Islands/03/2006 HA (SI06)
To characterize the redesigned C05 antibody, HDX-experiments were performed. The data show that the CDR3-loop exhibited the strongest HDX-perturbation in complex with SI06, as expected. Further, the interaction site on the paratope could was mapped to a location at the rim of the receptor-binding domain. These finding complement the predicted models created from computational and crystal structures.
*Marcus ended work on this project December 31, 2018
Mentors
Primary: Kevin Schey
Secondary: James E. Crowe, Jr. & Jens Meiler
Type of Trainee
Postdoctoral Fellow


©2026 Vanderbilt University ·
Site Development: University Web Communications