Prefrontal-Thalamic anatomical connectivity and executive cognitive function in schizophrenia
Monica Giraldo-Chica, Baxter P. Rogers, Stephen M. Damon, Bennett A. Landman, Neil D. Woodward. “Prefrontal-Thalamic anatomical connectivity and executive cognitive function in schizophrenia.” Biological Psychiatry, September 2017. http://dx.doi.org/10.1016/j.biopsych.2017.09.022
Full text: http://www.biologicalpsychiatryjournal.com/article/S0006-3223(17)32010-3/fulltext NIHMSID
Abstract
Background
Executive cognitive functions, including working memory, cognitive flexibility, and inhibition, are impaired in schizophrenia. Executive functions rely on coordinated information processing between the prefrontal cortex (PFC) and thalamus, particularly the mediodorsal (MD) nucleus. This raises the possibility that anatomical connectivity between the PFC and MD thalamus may be: 1) reduced in schizophrenia; and 2) related to deficits in executive function. The current investigation tested these hypotheses.
Methods
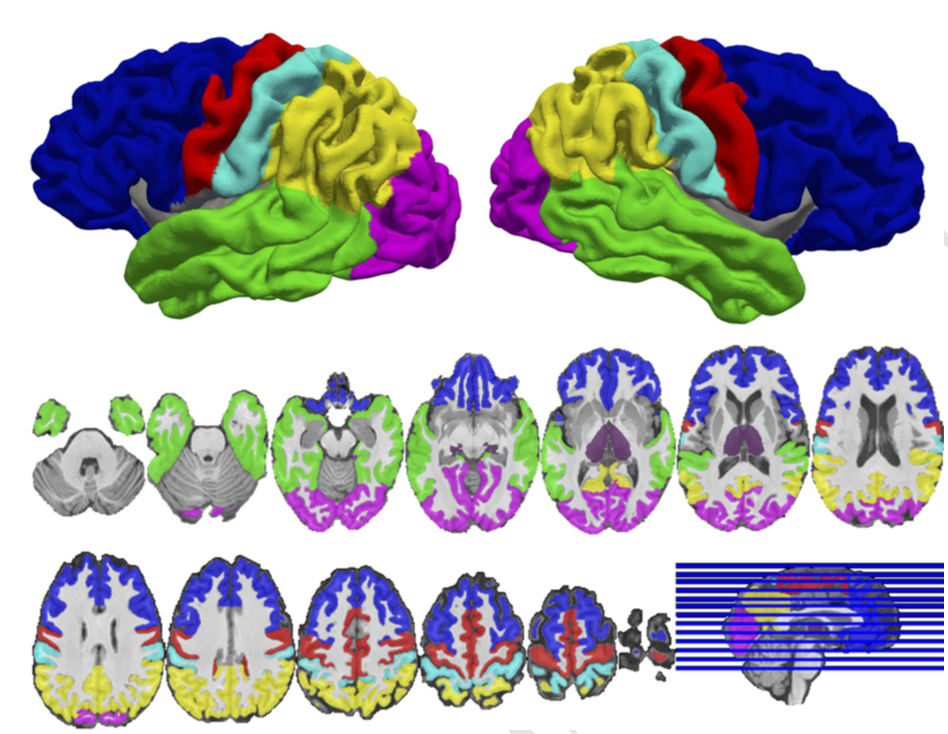
45 healthy subjects and 62 individuals with a schizophrenia spectrum disorder completed a battery of tests of executive function and underwent diffusion-weighted imaging (DWI). Probabilistic tractography was used to quantify anatomical connectivity between six cortical regions, including the PFC, and the thalamus. Thalamocortical anatomical connectivity was compared between healthy subjects and schizophrenia using region-of-interest and voxel-wise approaches, and the association between PFC-thalamic anatomical connectivity and severity of executive function impairment was examined in patients.
Results
Anatomical connectivity between the thalamus and PFC was reduced in schizophrenia. Voxel-wise analysis localized the reduction to areas of the MD thalamus connected to lateral PFC. Reduced PFC-thalamic connectivity in schizophrenia correlated with impaired working memory, but not cognitive flexibility and inhibition. In contrast to reduced PFC-thalamic connectivity, thalamic connectivity with somatosensory and occipital cortices was increased in schizophrenia.
Conclusions
The results are consistent with models implicating dysrupted PFC-thalamic connectivity in the pathophysiology of schizophrenia and mechanisms of cognitive impairment. PFC-thalamic anatomical connectivity may be an important target for pro-cognitive interventions. Further work is needed to determine the implications of increased thalamic connectivity with sensory cortex.
Figure