Multimodal neuroimaging in pediatric type 1 diabetes: a pilot multisite feasibility study of acquisition quality, motion, and variability
Leon Y. Cai, Costin Tanase, Adam W. Anderson, Karthik Ramadass, Francois Rheault, Chelsea A. Lee, Niral J. Patel, Sky Jones, Lauren M. LeStourgeon, Alix Mahon, Sumit Pruthi, Kriti Gwal, Arzu Ozturk, Hakmook Kang, Nicole Glaser, Simona Ghetti, Sarah S. Jaser, Lori C. Jordan, and Bennett A. Landman
Abstract
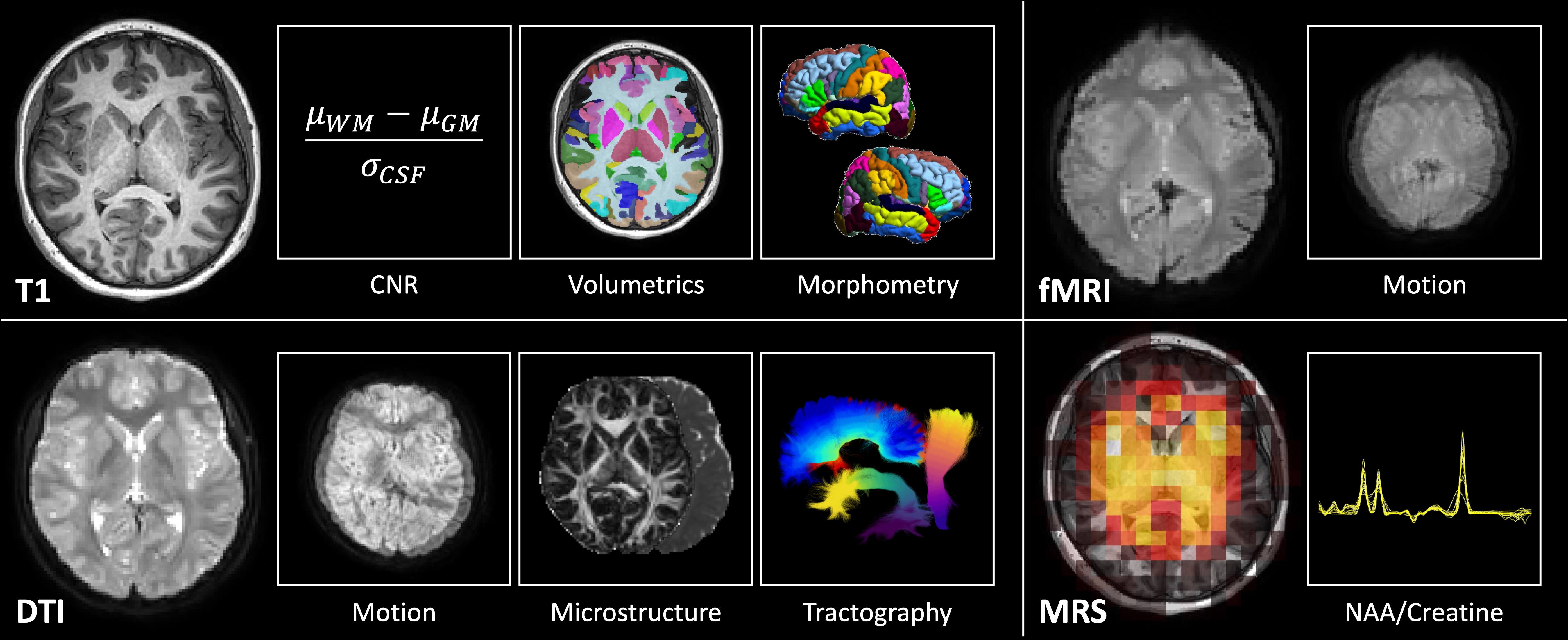
Type 1 diabetes (T1D) affects over 200,000 children and is associated with an increased risk of cognitive dysfunction. Prior imaging studies suggest the neurological changes underlying this risk are multifactorial, including macrostructural, microstructural, and inflammatory changes. However, these studies have yet to be integrated, limiting investigation into how these phenomena interact. To better understand these complex mechanisms of brain injury, a well-powered, prospective, multisite, and multimodal neuroimaging study is needed. We take the first step in accomplishing this with a preliminary characterization of multisite, multimodal MRI quality, motion, and variability in pediatric T1D. We acquire structural T1 weighted (T1w) MRI, diffusion tensor MRI (DTI), functional MRI (fMRI), and magnetic resonance spectroscopy (MRS) of 5-7 participants from each of two sites. First, we assess the contrast-to-noise ratio of the T1w MRI and find no differences between sites. Second, we characterize intervolume motion in DTI and fMRI and find it to be on the subvoxel level. Third, we investigate variability in regional gray matter volumes and local gyrification indices, bundle- wise DTI microstructural measures, and N-acetylaspartate to creatine ratios. We find the T1-based measures to be comparable between sites before harmonization and the DTI and MRS-based measures to be comparable after. We find a 5-15% coefficient of variation for most measures, suggesting ~150-200 participants per group on average are needed to detect a 5% difference across these modalities at 0.9 power. We conclude that multisite, multimodal neuroimaging of pediatric T1D is feasible with low motion artifact after harmonization of DTI and MRS.