AI Body Composition in Lung Cancer Screening: Added Value Beyond Lung Cancer Detection
Kaiwen Xu, Mirza S. Khan, Thomas Z. Li, Riqiang Gao, James G. Terry, Yuankai Huo, Thomas A. Lasko, John Jeffrey Carr, Fabien Maldonado, Bennett A. Landman, Kim L. Sandler
Paper: https://pubs.rsna.org/doi/epdf/10.1148/radiol.222937
Abstract
Background
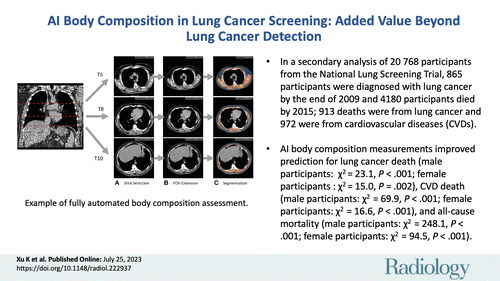
An artificial intelligence (AI) algorithm has been developed for fully automated body composition assessment of lung cancer screening noncontrast low-dose CT of the chest (LDCT) scans, but the utility of these measurements in disease risk prediction models has not been assessed.
Purpose
To evaluate the added value of CT-based AI-derived body composition measurements in risk prediction of lung cancer incidence, lung cancer death, cardiovascular disease (CVD) death, and all-cause mortality in the National Lung Screening Trial (NLST).
Materials and Methods
In this secondary analysis of the NLST, body composition measurements, including area and attenuation attributes of skeletal muscle and subcutaneous adipose tissue, were derived from baseline LDCT examinations by using a previously developed AI algorithm. The added value of these measurements was assessed with sex- and cause-specific Cox proportional hazards models with and without the AI-derived body composition measurements for predicting lung cancer incidence, lung cancer death, CVD death, and all-cause mortality. Models were adjusted for confounding variables including age; body mass index; quantitative emphysema; coronary artery calcification; history of diabetes, heart disease, hypertension, and stroke; and other PLCOM2012 lung cancer risk factors. Goodness-of-fit improvements were assessed with the likelihood ratio test.
Results
Among 20 768 included participants (median age, 61 years [IQR, 57–65 years]; 12 317 men), 865 were diagnosed with lung cancer and 4180 died during follow-up. Including the AI-derived body composition measurements improved risk prediction for lung cancer death (male participants: χ2 = 23.09, P < .001; female participants: χ2 = 15.04, P = .002), CVD death (males: χ2 = 69.94, P < .001; females: χ2 = 16.60, P < .001), and all-cause mortality (males: χ2 = 248.13, P < .001; females: χ2 = 94.54, P < .001), but not for lung cancer incidence (male participants: χ2 = 2.53, P = .11; female participants: χ2 = 1.73, P = .19).
Conclusion
The body composition measurements automatically derived from baseline low-dose CT examinations added predictive value for lung cancer death, CVD death, and all-cause death, but not for lung cancer incidence in the NLST.